
Post-operative delirium: a review of diagnosis and treatment strategies
Introduction
Delirium is not a new problem. Historians have been able to trace its appearance in the medical literature back to 1 AD (1). The term comes from the Latin word “delirare” which translates to mean “to deviate from the furrow” (2,3). In farming terms, patients who are delirious have gone astray from the straight path that was meant to be plowed. Despite acknowledgement of this condition for many years, the exact causes and best management remain elusive. In the last 2 decades, the medical literature has started to try to untangle some of the mystery surrounding delirium, yet it remains underdiagnosed and poorly managed in many institutions. Data suggests that delirium is the most common post-operative neurological complication among elderly patients (4). Therefore, it is imperative that clinicians become more adept at preventing and managing this problem in order to improve post-surgical outcomes.
Many reasons exist for the lack of appropriate identification and treatment of post-operative delirium (POD) including failure to obtain the patient’s prehospital level of functioning, an expectation that confusion among hospitalized elderly is normal, or simply a failure to include delirium in the differential. The failure to appropriately recognize and treat POD is causing significant harm to patients. There is clear evidence that delirium results in increased mortality, increased lengths of stay, increased institutionalization, and decreased functioning for patients (5-12). As such, it is essential that medical professionals deepen their understanding of POD and take steps to lessen the incidence of delirium and its impact on patients.
Delirium defined
The World Health Organization (WHO) has defined delirium as “an etiologically nonspecific organic cerebral syndrome characterized by concurrent disturbances of consciousness and attention, perception, thinking, memory, psychomotor behavior, emotion, and the sleep-wake schedule. The duration is variable and the degree of severity ranges from mild to very severe” (13). The American Psychiatric Association put forth a similar definition in its 5th edition of the Diagnostic and Statistical Manual (DSM-V). The DSM-V criteria for diagnosis of delirium includes a disturbance of attention, a disturbance in cognition, and an acute change from cognitive baseline which has not been previously established or is not caused directly by another medical condition (14).
Delirium is often thought to have two predominant forms: hyperactive and hypoactive. The hyperactive form is usually readily identified as the patient is often agitated, aggressive, refuses to cooperate with medical personnel, may be found pacing, and may pull out intravenous lines, catheters, or intubation tubes. Additionally, patients with the hyperactive form of delirium are prone to experiencing visual hallucinations, less commonly auditory hallucinations, and delusions (2,15). These hallucinations and delusions can be extremely distressing for patients and family, particularly if the patient believes that the medical staff is trying to harm him/her. Even after the resolution of delirium, some patients continue to believe that the nursing staff or their doctors was trying to injure or poison them and actually develop post-traumatic stress disorder from the experience (16).
In contrast to this hyperactive form of delirium, the hypoactive form is characterized by a decrease in psychomotor activity. Patients experiencing this form of delirium are often lethargic and may appear depressed. Often times, they will awaken when you call their name but then quickly return to sleeping. These patients are also confused, though the confusion may not be apparent unless the patient’s attention and thought process are investigated. Unfortunately, this form of delirium is commonly missed, disproportionately effects older patients, and is associated with poorer outcomes (2,15).
A third form of delirium, called the mixed form, is present in patients who exhibit features of both hyperactive and hypoactive delirium.
It is important to remember that patients with delirium have fluctuations throughout their course. It is not uncommon for the nursing staff to call a physician with concerns about the patient’s mental status and by the time the provider arrives, the patient appears to be fine. Agitation is often worse at night, such that a daytime medical team may get reports of a patient acting out in the evening but never actually witness these behaviors (17).
Pathophysiology
The exact mechanisms which lead to the development of delirium remain unclear. It is generally accepted that delirium is a result of the patient’s underlying vulnerabilities or risk factors combines with an outside stressor such as infection or surgery (11,15). Many different stressors have been suggested including neurotransmitter imbalance (particularly a cholinergic deficiency), inflammation, and electrolyte or metabolic derangements (18). Since the pathophysiology of delirium is still not well understood, it is important to identify the risk factors that are well known and can be acted upon.
Risk factors
It has been shown that delirium can be prevented (19). Therefore, it is important to be aware of patients’ modifiable risk factors so steps can be taken to optimize the patient, prior to elective surgery, with the hope of reducing the likelihood of a patient developing POD (2). Depending on the source used, risk factors vary though pre-existing cognitive impairment is often viewed as the greatest risk for development of delirium (5). Several recent studies have looked at the risk factors for development of delirium in cardiac and non-cardiac surgeries (Table 1). The risk factors found in both surgical groups are old age, cognitive impairment, decreased albumin, and weight loss (5,20-25).
Table 1
| Risk factor for delirium | Cardiac surgery (19-23) | Non-cardiac surgery (4,24) |
|---|---|---|
| Old age | X | X |
| Decreased albumin | X | X |
| Anemia | X | |
| Poor functional status | X | |
| Cognitive impairment | X | X |
| Increased comorbidities | X | |
| Pre-existing stroke | X | |
| History of atrial fibrillation | X | |
| Longer procedure time | X | |
| Electrolyte disturbance | X | |
| Weight loss | X | X |
| Two or more blood transfusions | X | |
| Depression | X | |
| Diabetes | X | |
| Current smoking | X | |
| Carotid artery disease | X | |
| Non-transfemoral approach (TAVR) | X |
X, risk factor.
Of note, using a non-transfemoral approach (transapical or transaortic) during transcatheter aortic valve replacement is associated with higher rates of delirium compared to when the procedure is done using the traditional transfemoral approach, and should be avoided when possible (22,25).
The United Kingdom’s National Institute for Health and Clinical Excellence’s (NICE) guidelines indicate that patients above the age of 65, those with cognitive impairment, and those with severe illness or hip fracture are at increased risk of developing POD (16). The American Geriatrics Society (AGS) put forth 22 risk factors for POD and states that patients with 2 or more risk factors are at increased risk of POD (16).
Risk prediction models
Several models have been proposed to predict an individual’s risk of developing POD. One of the best known models was developed by Marcantonio et al. for patients undergoing elective noncardiac surgery. Risk factors included in the model are age greater than 70, alcohol abuse, a score of less than 30 on a Telephone Interview for Cognitive Status, severe physical impairment as indicated by a Specific Activity Scale class IV, markedly abnormal electrolytes, aortic aneurysm surgery, and noncardiac thoracic surgery. All risk factors are given 1 point, except for aortic aneurysm surgery which receives 2 points. Patients receiving 1–2 points have an 11% risk of delirium while patients receiving 3 or more points have a 50% chance of developing delirium (11).
Rudolph et al. devised a model for predicting delirium after cardiac surgery. In this model, patients receive 1 point each for a previous stroke or TIA, abnormal albumin (<3.5 or >4.5 g/dL), and a score of greater than 4 on the Geriatric Depression Scale. Patients receive an additional point if their mini mental state examination (MMSE) score falls between 24 to 27 and an additional 2 points for an MMSE score of 23 or less. Using this model, a score of 1 is associated with a 43% risk of developing delirium, a score of 2 equates to a 60% risk, and a score of 3 or more equates to an 87% risk (26).
Inouye et al. also developed a prediction model for general medical patients which evaluates the patient for visual impairment, cognitive impairment, severe illness, and a high blood urea nitrogen/creatinine ratio. Patients with 3 or 4 of these risk factors were found to have an 83% chance of developing delirium in the hospital (27).
Outcomes
Cognitive outcomes
It is well documented that patients who experience delirium have long lasting adverse effects to their cognition (28-30). Levkoff et al. reported that in those patients who developed delirium, only 17.7% had complete resolution of delirium associated symptoms, including inattention and disorientation, at 6 months post hospital discharge (28). A 2012 study by Saczynski et al. similarly sought to determine the long-term consequences of delirium on cognition. The authors determined that patients who had experienced delirium were less likely to have returned to their preoperative cognitive baseline at 6 months. In fact, patients without delirium were typically back to preoperative cognitive baseline by 1 month after surgery, whereas patients who had developed delirium remained below their baseline 1 year postoperatively (29).
Most healthcare professionals who care for older patients acknowledge a link exists between delirium and dementia. In one study of patients undergoing cardiac surgery, 87% of those who developed dementia had experienced POD (31). Two additional studies, with an average follow-up of 4.1 years, also showed increased rates of dementia in patients who had previously suffered from delirium (62.5% vs. 8.1%) (12).
In a sort of “chicken or the egg” dilemma, it remains unclear if delirium is an early indicator of dementia or if delirium itself leads to dementia. Recent research has suggested that delirium may be associated with dementia in multiple ways. Delirium seems to indicate a vulnerable brain, may be the first notice of previously undocumented dementia, and creates neuronal damage that itself can result in dementia (32).
Increased institutionalization
Patients who develop delirium are known to require more assistance with activities of daily living (ADLs) both at hospital discharge and at 6 months post-discharge (10). As the ability to successfully perform one’s own ADLs is used a benchmark for a safe discharge home, it is not surprising that many patients with delirium are discharged to nursing facilities or long-term care. In a study of patients who underwent elective noncardiac surgery, 36% of patients with delirium were discharged to nursing facilities, as opposed to only 11% of patients whose hospitalization was not complicated by delirium (11). A more recent meta-analysis of 7 studies also demonstrated a greater than 3 fold increased risk of institutionalization among patients with delirium (33.4% vs. 10.7%) (12).
Increased length of stay
In healthcare systems where reimbursement is based on diagnosis, rather than days in the hospital and services used, increased lengths of stay can result in significant loss of revenue. Length of stay can be more than double in patients with delirium (11). Additionally, increased lengths of stay put the patient at increased risk of medical error, infection, and deconditioning.
Importance of preoperative screening
In a best practice statement from AGS, it has been recommended that patients undergoing surgery should undergo a preoperative assessment to look for delirium risk factors (33). The Association of Anaesthetists in Great Britain and Ireland has also issued a recommendation that elderly patients undergo delirium risk assessment prior to receiving anesthesia (16).
If POD is viewed as a form of acute brain failure, then it becomes clear that it is a significant complication in hospitalized patients. As such, it is important for patients to be aware of their risk for POD as part of the process of informed consent (16). In addition to being appropriate to disclose the risk of delirium for legal reasons, it is likely that a discussion about the risk of delirium prior to surgery will help the patient’s family cope better if the complication should occur postoperatively (34).
At the preoperative risk assessment, the healthcare professional should clearly document the patient’s current cognitive status and his/her ability to perform ADLs (33,35). Additionally, vision and hearing assessments should be conducted and patients should be informed of the importance of bringing any glasses or hearing aids with them to the hospital (35). Many patients and their family members fear losing expensive visual or auditory aids, and therefore leave these items at home. Therefore, it is essential for the provider to explain the value that these items have in sensory optimization and delirium prevention.
Diagnosis
In addition to using the definitions set forth by WHO and AGS, there are multiple tools to aid in the diagnosis of delirium.
The most commonly used screening tool in research is the Confusion Assessment Method (CAM). This is probably due to the fact that when compared to a psychiatrist’s diagnosis of delirium, the CAM has demonstrated high sensitivity and specificity (between 90–100%) (36). There are many forms of the CAM, including the long form which has 10 sections and the short form which has 4. While the long form is used in research, the short form is commonly used in clinical practice. The short form examines four domains—acuity/fluctuation, attention, thinking, and level of consciousness. When compared with other screening tools, the CAM is also the best predictor of increased length of stay and mortality (37). A systemic review of 25 prospective studies examined the utility of bedside testing in the assessment of delirium. The authors concluded that while many options exist, the CAM has the best evidence for its use and can typically be completed in 5 minutes or less (38).
Another screening tool is called the Intensive Care Delirium Screening Checklist (ICDSC). The ICDSC evaluates 8 domains which include level of consciousness, attention, orientation, hallucinations/delusions, changes in psychomotor functioning, inappropriate speech or mood, sleep-wake disturbances, and fluctuations in symptoms (39). In a meta-analysis of 4 studies evaluating the accuracy of the ICDSC, the pooled sensitivity of the ICDSC was 74% and the pooled specificity was 81.9% (40).
The 4AT is a newer screening tool which may be particularly advantageous when practitioners have limited time to evaluate the patient. This tool evaluates the patient for 4 hallmarks of delirium—alertness, orientation, attention, and acute change or fluctuating course. When compared with a geriatrician diagnosis, the 4T has been shown to have a sensitivity of 89.7% and specificity of 84.1% (41).
Even though many of these screening tools are short and fairly easy to perform, some providers still find them too time consuming. If that is the case, it has been suggested that assessing the patient’s orientation and asking them to perform an attention task may be a permissible substitute (18). Attention tasks which have been recommended are serial 7’s (1 error allowed), or naming the days of the week backwards (no errors allowed) or the months of the year backwards (1 error allowed) (15,18,35).
After a patient has been diagnosed with delirium, additional tools, such as the memorial delirium assessment scale (MDAS) can be used to determine the severity of the delirium. The MDAS uses 10 items to evaluate several components of delirium including awareness, orientation, short-term memory, attention, disorganized thinking, and change in psychomotor activity or sleep disturbance (42). The severity of delirium determined by the MDAS has been shown to correlate to outcomes, with more severe delirium being associated with greater rates of institutionalization and mortality at 6 months (43).
Work-up
Once a patient has been determined to be delirious, it is important to evaluate for any underlying causes that may have precipitated the POD. Figure 1 illustrates a framework to consider when searching for possible causes of POD. Several tests can be run to look for a source of infection including a complete blood panel, a clean catch urine sample, blood cultures, and a chest X-ray. Additional tests can include a renal panel, liver function tests, ammonia level, thyroid function tests, and glucose level (2,44).
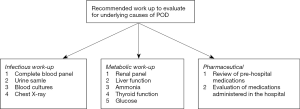
A careful review of the patient’s prehospital medication list, as well as medications the patient has received in the hospital, should also be performed (2,35). Antihistamines, benzodiazepines, opioids, and anticholinergic medications are all well-known precipitants of delirium (45,46). It is important to know about the patient’s compliance with his/her outside regimen and if he/she was taking any additional prescriptions or supplements. Alcohol history should also be obtained as both alcohol and benzodiazepine withdrawal can present with delirium.
It is also helpful to keep in mind that as the causes of delirium are multifactorial, providers should strive to treat all possible causes, not simply one (15,35).
Unless a patient has a focal neurological deficit, neuroimaging (computed tomography or magnetic resonance imaging) has been shown to offer little in delirious patients (2). Lumbar puncture should also not be used routinely unless there is a strong suspicion of meningitis (2).
Management strategies
Geriatric consultation
Geriatricians are trained to manage delirium, and so hospitals have begun using geriatric consultants to assist with or co-manage elderly surgical patients in an attempt to reduce incidence and sequelae of delirium. In a study that used geriatric consultation preoperatively or within 24 hours postoperatively for patients with hip fracture, there was a significant reduction in delirium in those patients who were seen by geriatricians (32% vs. 50% with usual care) (47). A meta-analysis by Wang also found reduced POD and better postoperative cognitive status in patients who received comprehensive geriatric care (48).
Multi-component interventions
As delirium is thought to be caused by multiple factors, it is no surprise that multi-component interventions have been found to reduce the incidence of delirium when compared to usual care (19,49). One of the most successful multi-component interventions is known as the Hospital Elder Life Program (HELP). HELP uses standardized protocols to target six risk factors (cognitive impairment, sleep deprivation, immobility, visual impairment, and dehydration) with the goal of delirium prevention and improvement in overall care (19). While the intervention has not been shown to have an effect on delirium severity, it has been shown to reduce delirium by 40% (19). The intervention does require a multi-disciplinary team and costs around $6,000 per case of delirium prevented (19). While this cost may deter many healthcare facilities from instituting a similar program, it is actually a savings considering that the one year cost of a single case of delirium has been estimated to be between $16,000 and $64,000 (50).
In 2010, the United Kingdom’s NICE developed recommendations for the prevention and management of delirium. The NICE guidelines expanded on the suggestions put forth by HELP (51). NICE suggests that patients should be evaluated for delirium risk on hospital admission with attention being paid to cognitive impairment, dehydration or constipation hypoxia, mobility impairments, infection, polypharmacy, pain, poor nutrition, sensory impairments, and sleep disturbance (51).
Anesthesia
The use of anesthesia has been linked with delirium and cognitive decline in the elderly (30). It has been suggested that regional anesthesia should be used rather than general anesthesia, but a 2016 review of 18 randomized controlled trials did not show a significant difference in incidence of delirium between the two forms of anesthesia (52). While the evidence remains limited, a meta-analysis of 2 studies did show that bispectral index (BIS) guided anesthesia was associated with lower rates of delirium than BIS-blinded anesthesia (49).
Pain management
It can be difficult manage pain, particularly in the perioperative period as both pain itself and opioids can cause delirium. It is recommended that a standing pain regimen be considered in older adults. Acetaminophen 1,000 mg, three times daily, is commonly used and has been shown to both reduce pain and the need for stronger medications (33,35).
Antipsychotics
The United States Food and Drug Administration has not approved any antipsychotics for use in delirium, but they are used frequently when non-pharmacological measures have not been effective in keeping the patient from being a danger to himself or others (53). While haloperidol is the most commonly used antipsychotic, many of the atypical antipsychotics have similar drug profiles and have been shown to be as effective in the management of delirium (54). Even though the effect of orally administered haloperidol on the QTc is rather small (less than 8 milliseconds), it is still recommended that patients receiving antipsychotics have electrocardiogram monitoring of the QTc (53).
While antipsychotics are used in the treatment of delirium, it remains unclear if they can be used prophylactically to prevent POD (15). A 2012 study did show delirium reduction when haloperidol was used prophylactically in patients undergoing non-cardiac surgery (55). In contrast, multiple studies of the prophylactic use of haloperidol for delirium prevention in elderly hip surgery patients have not shown any reduction in incidence of delirium (56,57).
There is consensus that antipsychotics should be discontinued (or weaned off over 3–5 days) once the delirium has resolved (2,44,53). Unfortunately, many patients remain on these medications after hospitalization. In a random sample of 300 geriatric patients who had received antipsychotics during their hospitalization, it was discovered that 48% of the patients were discharged on these medications (58). It is essential that a medication reconciliation is done prior to discharge and that the antipsychotic is stopped or clear communication occurs during the transition of care to indicate how the medication should be tapered.
Benzodiazepines
Since benzodiazepines are known to cause delirium, they are not recommended as treatment for delirium except in specific cases (59). In patients in whom delirium is the result of alcohol or sedative withdrawal, benzodiazepines are an appropriate choice. Benzodiazepines may also have to be used in patients with known Dementia with Lewy Bodies or Parkinson’s disease given their neuroleptic sensitivity (2,59). It is preferable to use a short acting benzodiazepine, such a lorazepam, in small doses in these cases (2). Patients who take regular benzodiazepines should continue these medications in the perioperative period, even if at a reduced dose, so as to prevent withdrawal-induced delirium. In patients who cannot take medications orally pre or post-surgery, benzodiazepines can be administered intravenously.
Ketamine
Based in part on research in children which has shown promise decreasing delirium, studies have looked at the intraoperative use of ketamine for the prevention of POD in older adults. The literature remains sparse with conflicting conclusions (60,61). At this time, ketamine is not recommended to prevent delirium and has been shown to cause an increase in hallucinations and nightmares (61).
Dexmedetomidine
The a-2 adrenergic agonist dexmedetomidine has gained popularity in ICUs in recent years. It is believed to have several benefits including reduced incidence of delirium and decreased opioid use (62). Dexmedetomidine has been shown to reduce delirium in both cardiac and noncardiac surgical patients. A recent study in China showed a significantly lower incidence of (noncardiac) POD in patients who received an IV infusion of dexmedetomidine (9% vs. 23%) (63). When compared with propofol after cardiac surgery, a meta-analysis of 8 randomized control trials did determine that dexmedetomidine was associated with a lower risk of delirium. Unfortunately, dexmedetomidine was also associated with a higher rate of bradycardia which may not be acceptable in cardiac patients (64). While postoperative dexmedetomidine seems to lower the incidence of delirium, intraoperative administration has not shown benefit (62).
Melatonin
Given the known sleep cycle disturbance in delirium, much hope has been placed on the use of melatonin for the management of delirium. A recent randomized control trial done in Japan, did show a decreased incidence of delirium for patients who were given the melatonin agonist Ramelteon prophylactically (65). However, a meta-analysis of 4 studies which examined the use of melatonin for delirium prevention showed mixed results. While a subgroup analysis did show that melatonin reduced the incidence of delirium in general surgical patients by 75%, no reduction in delirium incidence was seen in surgical patients (66). Another review of 3 studies found no clear evidence for the use of melatonin or melatonin agonists to reduce delirium (49). Instead of melatonin, it is advisable to use a nonpharmacological sleep protocol which includes a warm drink, guided meditation or relaxing music, and a back massage (15).
Follow-up
The last recommendation from NICE is that patients who have experienced delirium consider follow-up with a geriatric team. Given the association of delirium with dementia and other adverse outcomes, geriatric follow-up is likely to be beneficial in helping patients and families identify and manage residual deficits, as well as, hopefully reduce the risk of future occurrences of delirium (2).
Conclusions
POD is a significant problem facing both medical staff and surgical patients, particularly elderly ones. Despite being a known condition for many years, little is understood about the pathophysiology of the disorder and there is no specific cure. It is clear that preoperative evaluation allows for the identification of risk factors which make a patient more susceptible to the development of delirium. It is essential to identify both modifiable and non-modifiable risk factors so that steps can be taken to reduce the likelihood of delirium. Identification of risk factors in preoperative evaluations allows patients to understand their chances of developing delirium as part of the process of informed consent. In the hospital setting, there are a number of validated tools that identify the presence of delirium. Prevention is the best approach to delirium; however, once it has developed, both geriatric consultation and multi-intervention strategies have been shown to be effective. Despite the routine use of antipsychotics for delirium, no current drug regimen exists to correct the problem. Given the known poor outcomes associated with delirium, it is important that researchers continue to look for the best ways to both prevent delirium and treat it when it does occur.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Moises Auron, Christopher Whinney) for the series “Update in Perioperative Medicine” published in Journal of Xiangya Medicine. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.01.03). The series "Update in Perioperative Medicine" was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Adamis D, Treloar A, Martin FC, et al. A brief review of the history of delirium as a mental disorder. Hist Psychiatry 2007;18:459-69. [Crossref] [PubMed]
- Saxena S, Lawley D. Delirium in the elderly: a clinical review. Postgrad Med J 2009;85:405-13. [Crossref] [PubMed]
- Dictionary OE. Delirium. Oxford English Dictionary 2014.
- Sieber FE, Barnett SR. Preventing postoperative complications in the elderly. Anesthesiol Clin 2011;29:83-97. [Crossref] [PubMed]
- Robinson TN, Raeburn CD, Tran ZV, et al. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg 2009;249:173-8. [Crossref] [PubMed]
- Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006;35:350-64. [Crossref] [PubMed]
- McAvay GJ, Van Ness PH, Bogardus ST Jr, et al. Older adults discharged from the hospital with delirium: 1-year outcomes. J Am Geriatr Soc 2006;54:1245-50. [Crossref] [PubMed]
- Zhang Z, Pan L, Ni H. Impact of delirium on clinical outcome in critically ill patients: a meta-analysis. Gen Hosp Psychiatry 2013;35:105-11. [Crossref] [PubMed]
- Lin SM, Huang CD, Liu CY, et al. Risk factors for the development of early-onset delirium and the subsequent clinical outcome in mechanically ventilated patients. J Crit Care 2008;23:372-9. [Crossref] [PubMed]
- Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc 2011;59:S301-4. [Crossref] [PubMed]
- Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA 1994;271:134-9. [Crossref] [PubMed]
- Witlox J, Eurelings LS, de Jonghe JF, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010;304:443-51. [Crossref] [PubMed]
- Organization WH. International Statistical Classification of Diseases and Related Health Problems (ICD). Available online: http://apps.who.int/classifications/icd10/browse/2010/en
- Association AP. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Available online: http://ajp.psychiatryonline.org/article.aspx?articleID=158714%5Cn
- Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol 2009;5:210-20. [Crossref] [PubMed]
- Tomlinson JH, Partridge JS. Preoperative discussion with patients about delirium risk: are we doing enough? Perioper Med (Lond) 2016;5:22. [Crossref] [PubMed]
- Brown TM, Boyle MF. Delirium. BMJ 2002;325:644-7. [Crossref] [PubMed]
- Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911-22. [Crossref] [PubMed]
- Inouye SK, Bogardus ST Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340:669-76. [Crossref] [PubMed]
- Cereghetti C, Siegemund M, Schaedelin S, et al. Independent Predictors of the Duration and Overall Burden of Postoperative Delirium After Cardiac Surgery in Adults: An Observational Cohort Study. J Cardiothorac Vasc Anesth 2017;31:1966-73. [Crossref] [PubMed]
- Soundhar A, Udesh R, Mehta A, et al. Delirium Following Transcatheter Aortic Valve Replacement: National Inpatient Sample Analysis. J Cardiothorac Vasc Anesth 2017;31:1977-84. [Crossref] [PubMed]
- Abawi M, Nijhoff F, Agostoni P, et al. Incidence, Predictive Factors, and Effect of Delirium After Transcatheter Aortic Valve Replacement. JACC Cardiovasc Interv 2016;9:160-8. [Crossref] [PubMed]
- Neerland BE, Krogseth M, Juliebø V, et al. Perioperative hemodynamics and risk for delirium and new onset dementia in hip fracture patients; A prospective follow-up study. PLoS One 2017;12:e0180641. [Crossref] [PubMed]
- Lin Y, Chen J, Wang Z. Meta-analysis of factors which influence delirium following cardiac surgery. J Card Surg 2012;27:481-92. [Crossref] [PubMed]
- Bagienski M, Kleczynski P, Dziewierz A, et al. Incidence of Postoperative Delirium and Its Impact on Outcomes After Transcatheter Aortic Valve Implantation. Am J Cardiol 2017;120:1187-92. [Crossref] [PubMed]
- Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation 2009;119:229-36. [Crossref] [PubMed]
- Inouye SK, Viscoli CM, Horwitz RI, et al. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med 1993;119:474-81. [Crossref] [PubMed]
- Levkoff SE. Delirium. Archives of Internal Medicine 1992;152.
- Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med 2012;367:30-9. [Crossref] [PubMed]
- Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet 1998;351:857-61. [Crossref] [PubMed]
- Lingehall HC, Smulter NS, Lindahl E, et al. Preoperative Cognitive Performance and Postoperative Delirium Are Independently Associated With Future Dementia in Older People Who Have Undergone Cardiac Surgery: A Longitudinal Cohort Study. Crit Care Med 2017;45:1295-303. [Crossref] [PubMed]
- Fong TG, Davis D, Growdon ME, et al. The interface between delirium and dementia in elderly adults. The Lancet Neurology 2015;14:823-32. [Crossref] [PubMed]
- American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults. Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg 2015;220:136-48.e1. [Crossref] [PubMed]
- Partridge JS, Martin FC, Harari D, et al. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry 2013;28:804-12. [Crossref] [PubMed]
- Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg 2011;112:1202-11. [Crossref] [PubMed]
- Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med 1990;113:941-8. [Crossref] [PubMed]
- Tomasi CD, Grandi C, Salluh J, et al. Comparison of CAM-ICU and ICDSC for the detection of delirium in critically ill patients focusing on relevant clinical outcomes. J Crit Care 2012;27:212-7. [Crossref] [PubMed]
- Wong CL, Holroyd-Leduc J, Simel DL, et al. Does this patient have delirium?: value of bedside instruments. JAMA 2010;304:779-86. [Crossref] [PubMed]
- Bergeron N, Dubois MJ, Dumont M, et al. Intensive Care Delirium Screening Checklist: evaluation of a new screening tool. Intensive Care Med 2001;27:859-64. [Crossref] [PubMed]
- Gusmao-Flores D, Salluh JI, Chalhub RA, et al. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care 2012;16:R115. [Crossref] [PubMed]
- Bellelli G, Morandi A, Davis DH, et al. Validation of the 4AT, a new instrument for rapid delirium screening: a study in 234 hospitalised older people. Age Ageing 2014;43:496-502. [Crossref] [PubMed]
- Breitbart W, Rosenfeld B, Roth A, et al. The Memorial Delirium Assessment Scale. J Pain Symptom Manage 1997;13:128-37. [Crossref] [PubMed]
- Marcantonio E, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc 2002;50:850-7. [Crossref] [PubMed]
- Reuben DB HKP. Delirium. Geriatrics at Your Fingertips. Massachusetts: Blackwell Science, 2002.
- By the American Geriatrics Society Beers Criteria Update Expert P. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 2015;63:2227-46. [Crossref] [PubMed]
- Alagiakrishnan K, Wiens CA. An approach to drug induced delirium in the elderly. Postgrad Med J 2004;80:388-93. [Crossref] [PubMed]
- Marcantonio ER, Flacker JM, Wright RJ, et al. Reducing Delirium After Hip Fracture: A Randomized Trial. Journal of the American Geriatrics Society 2001;49:516-22. [Crossref] [PubMed]
- Wang Y, Tang J, Zhou F, et al. Comprehensive geriatric care reduces acute perioperative delirium in elderly patients with hip fractures: A meta-analysis. Medicine (Baltimore) 2017;96:e7361. [Crossref] [PubMed]
- Siddiqi N, Harrison JK, Clegg A, et al. Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst Rev 2016;3:CD005563. [PubMed]
- Leslie DL, Marcantonio ER, Zhang Y, et al. One-year health care costs associated with delirium in the elderly population. Arch Intern Med 2008;168:27-32. [Crossref] [PubMed]
- Yue J, Tabloski P, Dowal SL, et al. NICE to HELP: operationalizing National Institute for Health and Clinical Excellence guidelines to improve clinical practice. J Am Geriatr Soc 2014;62:754-61. [Crossref] [PubMed]
- Bryson GL, Wyand A. Evidence-based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction. Can J Anaesth 2006;53:669-77. [Crossref] [PubMed]
- Thom RP, Mock CK, Teslyar P. Delirium in hospitalized patients: Risks and benefits of antipsychotics. Cleve Clin J Med 2017;84:616-22. [Crossref] [PubMed]
- Lonergan E, Britton AM, Luxenberg J, et al. Antipsychotics for delirium. Cochrane Database Syst Rev 2007;CD005594. [PubMed]
- Wang W, Li HL, Wang DX, et al. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial*. Crit Care Med 2012;40:731-9. [Crossref] [PubMed]
- Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc 2005;53:1658-66. [Crossref] [PubMed]
- Vochteloo AJ, Moerman S, van der Burg BL, et al. Delirium risk screening and haloperidol prophylaxis program in hip fracture patients is a helpful tool in identifying high-risk patients, but does not reduce the incidence of delirium. BMC Geriatr 2011;11:39. [Crossref] [PubMed]
- Loh KP, Ramdass S, Garb JL, et al. From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med 2014;9:802-4. [Crossref] [PubMed]
- Aarsland D, Perry R, Larsen JP, et al. Neuroleptic sensitivity in Parkinson's disease and parkinsonian dementias. J Clin Psychiatry 2005;66:633-7. [Crossref] [PubMed]
- Hudetz JA, Patterson KM, Iqbal Z, et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2009;23:651-7. [Crossref] [PubMed]
- Avidan MS, Maybrier HR, Abdallah AB, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 2017;390:267-75. [Crossref] [PubMed]
- Deiner S, Luo X, Lin HM, et al. Intraoperative Infusion of Dexmedetomidine for Prevention of Postoperative Delirium and Cognitive Dysfunction in Elderly Patients Undergoing Major Elective Noncardiac Surgery: A Randomized Clinical Trial. JAMA Surg 2017;152:e171505. [Crossref] [PubMed]
- Su X, Meng Z-T, Wu X-H, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893-902. [Crossref] [PubMed]
- Liu X, Xie G, Zhang K, et al. Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. J Crit Care 2017;38:190-6. [Crossref] [PubMed]
- Hatta K, Kishi Y, Wada K, et al. Preventive effects of ramelteon on delirium: a randomized placebo-controlled trial. JAMA Psychiatry 2014;71:397-403. [Crossref] [PubMed]
- Chen S, Shi L, Liang F, et al. Exogenous Melatonin for Delirium Prevention: a Meta-analysis of Randomized Controlled Trials. Mol Neurobiol 2016;53:4046-53. [Crossref] [PubMed]
Cite this article as: Cunningham J, Kim LD. Post-operative delirium: a review of diagnosis and treatment strategies. J Xiangya Med 2018;3:8.


