
Aorto-esophageal fistula after thoracic endovascular aortic repair
Introduction
Thoracic endovascular aortic aneurysm repair (TEVAR) was first approved for use in the US in 2005, and it has been an appealing alternative to the traditional open aortic aneurysm repair because of the comparatively better early outcomes (1). Major complications of TEVAR that typically necessitate open distal aortic repair include endoleak, aortic fistula, infection, device collapse or migration, and continued expansion of the aneurysm sac (1). Among these complications, aorto-esophageal fistula (AEF) is a rare and life-threatening complication.
We experienced a case of AEF with sepsis. Because of patient’s age and poor general condition, she was not a candidate for open surgery. Here we report our unique solution to AEF using an esophageal stent to cover an opening in the esophagus to the fistula.
Case presentation
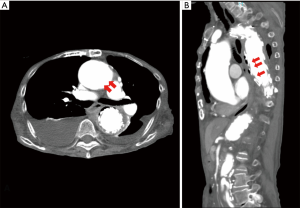
An 88-year-old woman visited our hospital presenting with appetite loss and chest pain. She was diagnosed with sepsis and was admitted. Seven months prior to admission, she had TEVAR for thoracic aortic aneurysm in another hospital. Her past history also included coronary artery bypass surgery and endovascular aortic repair for iliac artery. After TEVAR, she refused to visit the hospital for 6 months. Laboratory data at admission showed elevated white blood cells (WBC) counts of 12,400 µL and elevated C-reactive protein (CRP) of 19.29 mg/dL. Klebsiella pneumoniae was isolated in all 4 of 4 bottles of blood culture. Contrast chest CT scan revealed fluid congestion in mediastinum and free air around the stent-graft (Figure 1). Gastric fiberscopy revealed AEF in the middle intrathoracic esophagus, about 10 mm in diameter. The pus was flowing into the esophagus from the mediastinum (Figure 2). Klebsiella pneumoniae was also isolated from the pus. Complete repair by open surgery was high risk due to her advanced age and history of multiple cardiovascular surgeries, including coronary artery bypass surgery and endovascular stent grafting of the common iliac artery.
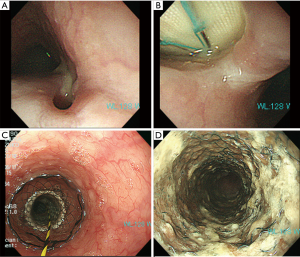
Oral intake was prohibited. She was on hyperalimentation and Ceftriaxone (CTRX) 2 g/day for 4 weeks. She was mentally alert and wished to eat her favorite foods. She underwent gastric fiberscopy again which revealed that the AEF had become more than double in size (Figure 2). Aortic stent-graft was prominent through such opening. In order to grant her wish to eat and drink again, we implanted a Niti-S esophageal stent (Taewoong Medical, Seoul, South Korea) 18 mm × 100 mm partial covered type to cover the entry to the fistula. She underwent prandial rehabilitation for 5 weeks. As a result, she was able to eat normally again and was thus discharged from the hospital.
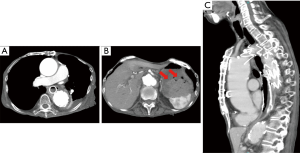
Seventeen weeks after the surgery she suddenly vomited blood and fell into hemorrhagic shock. Body CT scan showed blood in the stomach, however did not show any active bleeding (Figure 3). After blood transfusion she recovered from shock and became stable. Ten days after re-admission, gastric fiberscopy did not show any bleeding, and the esophageal stent was not dislocated (Figures 2,3). Fourteen days after re-admission she displayed sudden cyanosis followed by cardiac arrest and passed away. The hemoglobin level was 4.4, which suggested that she died of hemorrhagic shock.
Discussion
AEF is a rare post-TEVAR complication. It has been reported that the incidence of AEF post TEVAR is 1.5–1.9% (2-4). Aortic fistulas are difficult to treat, and patients rarely survive without treatment. The mechanism by which AEF develops is not known. Coselli et al. wrote in their review that fistulas develop after TEVAR because of the radial pressure the endograft exerts on nearby structures, coverage of arteries that provide blood flow to the esophagus (which could cause ischemic necrosis), a perforation, an infection, or erosion due to either an endoleak or infection-related pseudoaneurysm (1). Our case patient had refused to visit the hospital after TEVAR for 6 months, even though she was instructed by her previous hospital to do so. Therefore it is not known how she developed AEF. Since she presented with severe mediastinitis and sepsis, it is highly likely that AEF was worsened by the infection. The question was how she developed mediastinitis. The mediastinitis was caused by either aortic stent-graft infection or by esophageal perforation. Aortic stent-graft infection is rare, and the incidence is only 0.5–1% (5). It is more likely that the mediastinitis was caused by esophageal perforation as the result of swallowing of fish bone, chicken bone, or some other such hard item.
Esophageal stent is commonly used for esophageal stenosis such as esophageal cancer. It is typically constructed of nickel and titanium. Potential bleeding due to the friction between the two metal stents was of concern. Another concern was possible dislocation or migration of the stent, because the patient’s esophagus had an elastic surface unlike esophageal cancer. However, the patient and her family strongly desired to allow oral intake and for her to go home. Esophageal stent was subsequently inserted after obtaining informed consent. The patient would not have survived without such esophageal stent intervention, since the opening in the esophagus had grown significantly larger even though she did not eat for 4 weeks, and the mediastinitis was not controlled with antibiotics. It became necessary to repair the significantly enlarged opening in order to prevent the esophageal mucus or saliva from flowing into the mediastinum.
There are only three case reports in the medical literature that utilized esophageal stent to repair AEF developed after TEVAR. Onodera et al. used a covered self-expanding esophageal stent to repair AEF which developed 2 years after TEVAR, but the patient died of sepsis 34 days later (6). Tao et al. used Niti-S stent to tamponade the bleeding point of AEF secondary to TEVAR, but the stent migrated because of continuous vomiting so that they had to insert another stent (7). This patient died of myocardial infarction 8 months later. Eggebrecht et al. also used self-expanding esophageal stent for two patients of AEF secondary to TEVAR, but they died of mediastinitis in 5 weeks and 10 months (8). We used Niti-S stent with distal flare to prevent migration, and it was successfully inserted without dislocation. Nonetheless, none of the cases survived beyond one year with esophageal stent. It is thus only a temporal repair to close the fistula so that the patient can enjoy eating again, living a normal life at home with family, and significantly improve quality of life (QOL).
The cause of patient’s death points to hemorrhagic shock since the hemoglobin level dropped to one-third. This further points to the patient having aortic bleeding since she had sudden cyanosis and cardiac arrest. It is thus uncertain as yet whether the bleeding occurred due to friction between the two stents, or because the artery was damaged by prolonged mediastinitis.
Conclusions
Esophageal stent is shown to be an alternative treatment option for patients of AEF secondary to TEVAR who cannot undergo open surgery. Although esophageal stent treatment is generally not totally complete, it is shown a favored treatment option for those patients who wish to enjoy eating and drinking normally, enjoy and live a normal life, with improved QOL as long as possible.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2018.01.02). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coselli JS, Spiliotopoulos K, Preventza O, et al. Open aortic surgery after thoracic endovascular aortic repair. Gen Thorac Cardiovasc Surg 2016;64:441-9. [Crossref] [PubMed]
- Czerny M, Eggebrecht H, Sodeck G, et al. New insights regarding the incidence, presentation and treatment options of aorto-oesophageal fistulation after thoracic endovascular aortic repair: the European Registry of Endovascular Aortic Repair Complications. Eur J Cardiothorac Surg 2014;45:452-7. [Crossref] [PubMed]
- Eggebrecht H, Mehta RH, Dechene A, et al. Aortoesophageal fistula after thoracic aortic stent-graft placement: a rare but catastrophic complication of a novel emerging technique. JACC Cardiovasc Interv 2009;2:570-6. [Crossref] [PubMed]
- Grabenwöger M, Alfonso F, Bachet J, et al. Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg 2012;42:17-24. [Crossref] [PubMed]
- Sharif MA, Lee B, Lau LL, et al. Prosthetic stent graft infection after endovascular abdominal aortic aneurysm repair. J Vasc Surg 2007;46:442-8. [Crossref] [PubMed]
- Onodera M, Inoue Y, Fujino Y, et al. A case of secondary aortoesophageal fistula inserted a covered self-expanding esophageal stent to control gastrointestinal bleeding. Case Rep Gastrointest Med 2013;2013:857135.
- Tao M, Schlomovits E, Darling G, et al. Secondary aorto-esophageal fistula after thoracic aortic aneurysm endovascular repair treated by covered esophageal stenting. World J Clin Cases 2016;4:233-7. [Crossref] [PubMed]
- Eggebrecht H, Baumgart D, Radecke K, et al. Aortoesophageal fistula secondary to stent-graft repair of the thoracic aorta. J Endovasc Ther 2004;11:161-7. [Crossref] [PubMed]
Cite this article as: Harada Y, Makiyama H, Horita H, Hirota J. Aorto-esophageal fistula after thoracic endovascular aortic repair. J Xiangya Med 2018;3:5.


