
Personalized therapy in craniopharyngioma—novel perspectives and limitations
Introduction
Craniopharyngiomas are embryonic, histologically benign malformations of the sellar/parasellar region. Despite high survival rates (87% to 95% 20-yr overall survival in childhood-onset craniopharyngioma), quality of life is impaired in most long-term survivors due to sequelae caused by the anatomical proximity of the tumor to optic structures and hypothalamic–pituitary axes. Incidence rate is 0.17 to 0.2 cases per 100,000 people (1). A bimodal age distribution with peak incidence rates in children of ages 5 to 14 and adults of ages 50 to 74 years is known. No gender-related differences have been reported. Histologically two subtypes have been recognized, the adamantinomatous craniopharyngioma (aCP) and the papillary craniopharyngioma (pCP).
ACP is recognized by the presence of squamous epithelium disposed in cords, nodules and irregular trabeculae bordered by palisaded columnar epithelium. These islands of densely grouped cells are in close contact with cohesive aggregates of squamous cells described as stellate reticulum. Nodules of “wet keratin” representing remnants of pale nuclei embedded within an eosinophilic keratinous mass are observed in either the compact or looser areas. Cystic cavities containing squamous debris are lined by flattened epithelium. Granulomatous inflammation associated with cholesterol clefts and giant cells may be detectable, but this is more typical for xanthogranuloma. Piloid gliosis with abundant Rosenthal fibers is often seen at the infiltrative interface of the tumor and should not be mistaken for pilocytic astrocytoma.
PCP is almost exclusively diagnosed in adults (14 to 50% of adult cases and only up to 2% in pediatric cohorts). Macroscopically, it presents as solid mass. Mixed forms with cystic and solid components have been described. Calcifications are rare and the cyst fluid is usually viscous due to high lipid concentration and yellow (machine oil fluid). Whereas infiltration of adjacent brain tissue by neoplastic epithelium is frequently observed in aCP, this finding is rare in pCP. Microscopically, it is composed of pseudopapillae formed by mature squamous epithelial cells and of an anastomosing fibrovascular stroma without palisading of cells or stellate reticulin (2).
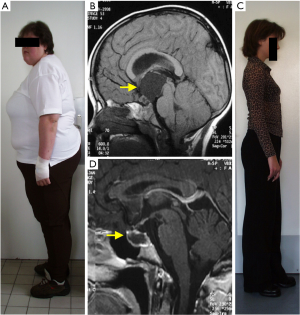
The currently recommended management of craniopharyngiomas of both histological types remains challenging. Therapeutic options include surgery combined or not with radiotherapy. However, craniopharyngiomas frequently present with aggressive growth behaviour with recurrences difficult to treat. Furthermore, craniopharyngiomas are associated with severe long-term morbidity (mainly involving endocrine, hypothalamic, visual, neuropsychologic sequelae) and mortality, associated with the disease and/or treated-related lesions of critical structures (3,4) (Figure 1).
Recent findings for molecular pathology of craniopharyngiomas have led to the development of therapies molecularly targeted to the underlying pathogenesis and aiming to improve prognosis in CP. As there have been important advances in this field, we summarise them with special regard to a recent publication of Brastianos et al. (5).
pCP
Until recently, molecular pathogenesis leading to pCP had remained less understood. Several studies in pCPs reported on the presence of activating mutations in BRAF (V600E). The observed prevalence rates range from 81% to 100% (6-9). Mutations other than those in exon 3 of CTNNB1 have not been reported (6-8). The lack of genetic complexity seen in these sellar masses is characteristic for their benign behaviour. A novel BRAF V600E mutation-specific antibody (VE1) might open a new perspective on diagnostics and differentiation between aCP and pCP (9). However, low antibody specificity in pituitary tissue should be kept in mind (10). Personalized, targeted pharmaceutical therapy with agents directed against the BRAF (V600E) mutation opens new therapeutic perspectives.
The observation that the majority of pCPs harbours a mutation of BRAF (V600E) provides the therapeutic option of using existing drugs for the therapy of currently treatment-refractory pCP cases. In other malignancies positive for BRAF (V600E) mutations, pharmaceutical agents that specifically inhibit mutant BRAF (V600E) are effective. The addition of trametinib, an MEK inhibitor acting on the MAPK pathway, reduces the risk of cutaneous squamous-cell carcinoma, which has been reported as sequelae after targeted therapy using BRAF inhibitors (11).
Recent publications reported on clinically significant tumour volume reduction due to BRAF (V600E) targeted therapy in pCP cases. Aylwin et al. (12) reported on a patient with visual deterioration due to a recurrence of pCP after three surgical interventions and external photon irradiation. A BRAF (V600E) mutation was detected and the patient was treated with vemurafenib. Within two weeks of targeted therapy, a significant reduction in tumour volume and stabilization of visual function were achieved. A good response was observed in neuroradiological monitoring. Due to infectious complications, targeted therapy was interrupted after three months but the tumor recurred within six weeks and targeted therapy with vemurafenib was restarted. Tumor progression was stabilized again. Seven months later, tumor progression was observed again.
Brastianos and colleagues (5) reported on a second case with multiple recurrent pCP harboring a BRAF (V600E mutation). Multiple surgical interventions did not result in controlling cystic tumour progression. The patient developed panhypopituitarism and bilateral optic neuropathy. After dabrafenib monotherapy for 17 days, both solid and cystic components decreased by 50% and 70%, respectively. In order to reduce the likelihood of resistance to BRAF inhibition, additional medication with a MEK inhibitor (trametinib) initiated. This treatment resulted in volume reduction of solid and cystic components by 85% and 81%, respectively. After transsphenoidal endoscopic tumor resection and photon irradiation, the patient remained in complete remission.
aCP
The majority of mutations in aCP are located in regulatory amino acids encoded by exon 3 of the CTNNB1 gene. Due to these mutations, a mutant form of beta-catenin is expressed resulting in enhanced degradation resistance, which leads to accumulation of beta-catenin and subsequent activation of the WNT pathway. Typically, cell clusters with activated WNT pathway can be detected in human aCP (13). Pathogenesis downstream the WNT/beta-catenin signal pathways has been analysed using gene expression profiling studies of human aCP, showing that the activation of these pathways could be specifically inhibited by targeted therapy. However, clinical studies to prove this hypothesis are still lacking, partly due to the fact that via WNT/beta-catenin signal pathways several physiological processes are regulated. Pre-clinical models of human aCP help to assess the effects of the WNT/beta-catenin, EGF, and Claudin-1 pathways on invasion and migration (14). A genetically engineered mouse model (GEMM) has been established by expressing a mutant form of beta-catenin that is resistant to degradation in undifferentiated embryonic precursors of the pituitary gland (13) and functionally equivalent to that identified in human aCP, therefore the molecular etiology in this GEMM is similar to human aCP (15).
A different GEMM has been established by targeting the expression of oncogenic beta-catenin into adult SOX2-positive pituitary stem cells (16). The resulting tumours resemble with human aCP. However, similar to the embryonic model, these tumours do not infiltrate the brain or visual pathway and lack some histological features of the human aCP (e.g., palisading epithelium and wet keratin). There is increasing literature of further specific targets in aCP such as sonic hedgehog, angiogenesis, EGFR pathways, which still needs to be tested in clinical trials.
Perspectives and limitations of targeted therapy in CP
For CP with favourable location i.e. without hypothalamic involvement, the therapeutic strategy of choice, especially at primary diagnosis, is an attempt at gross-total resection with preservation of visual and hypothalamic function (1,17-19). For unfavourably localized tumours—those too close to the hypothalamus and/or the optic chiasm—a limited surgical resection followed by radiation therapy should be preferred in order to preserve the integrity and to avoid further damage to hypothalamic and optic structures (20,21). Recently, several algorithms have been published recommending risk-adapted treatment strategies aligning to the above-mentioned goals. Puget et al. (18,22) published an algorithm for surgical strategies in CP patients, which recommends a hypothalamus-sparing surgical treatment based on neuroradiological grading of hypothalamic involvement in preoperative magnetic resonance imaging (MRI) (22). Patients neurosurgically treated according to this strategy using a hypothalamus-sparing approach presented with lower prevalence of severe obesity and similar recurrence rates when compared with patients treated by radical complete resection (28% versus 54%, respectively) (18). The authors are reporting on good efficacy and tolerability of such a hypothalamus-sparing approach by comparing patients treated by the same surgical team at a single institution, and thus eliminating the potential bias of surgical experience on outcome prognosis. It has to be pointed out, that although the “hypothalamus-sparing surgery” lead to an increase of “normal weight condition” from 17% to 38%, the rate of significant weight gain remained 62% with nearly half of all CP patients developing morbid obesity. Müller et al. (23,24) reported on outcome after CP related to surgical strategies based on pre and post-surgical grading of hypothalamic involvement/damage in MRI. The neuro radiological assessment of the suprasellar extension of CP towards the mammillary bodies is the major criteria for their grading of anterior or posterior hypothalamic involvement/lesion. Patients with surgical lesions affecting posterior hypothalamic areas had a higher risk for postsurgical development of obesity and reduced self-assessed quality of life during long-term prospective follow-up. Mallucci et al. (25) suggested a two-staged surgical approach with initial relief of cystic pressure by cyst fenestration and/or catheter implantation. According to their experience, down-staging the risk grade and improving outcome in appropriate cases could be achieved.
Treatment with targeted therapy using agents such as dabrafenib in BRAF mutated eCP fit in this concept of risk-adapted, hypothalamus-sparing strategies. Effective preoperative tumor shrinkage due to targeted therapy might support more favourable surgical strategies to prevent risks of hypothalamic damage. Especially in pCP with persistent tendency towards progression even after several surgical interventions, targeted therapy with dabrafenib combined with an MEK inhibitor (trametinib) offers a therapeutic option with considerable perspective of improving the course of disease. Of special interest is, that the authors detected BRAFV600E in peripheral blood of the pCP patient. Initial detection of BRAFV600E mutation, could offer the opportunity to facilitate early implementation of neoadjuvant-targeted therapy. A possible caveat mentioned by the authors is that the DNA may have been released as a consequence of the multiple surgical procedures, the concurrent drug treatment, or both. In aCP, the recent availability of an animal model to test pharmaceutical agents for therapeutic effectiveness in terms of tumor shrinkage also offers novel perspectives with the goal to improve prognosis. Theses preclinical studies are of special value because molecular targeted therapies (as currently conducted in pCP) are mainly considered as salvage therapy for relapsed patients.
However, several reports have shown that initial preoperative hypothalamic involvement in aCP has major apriori impact on long-term prognosis regardless of chosen therapeutic strategies. Based on the observation of hypothalamic involvement due to finger-like protrusions of craniopharyngioma tissue into hypothalamic areas, it seems questionable whether effective tumor shrinkage in CP patients with such initial hypothalamic involvement would result in an altered (i.e., improved) long-term prognosis, which is more likely if involvement is caused by hypothalamic compression. It seems more likely that in many CP patients with initial hypothalamic involvement long-term prognosis is determined by neuroendocrine pathologies, which cannot be improved or even cured by tumor shrinkage but only aggravated by surgical interventions. Accordingly, it is a matter of debate whether targeted therapy could change this frustrating therapeutic situation for CP patients with initial hypothalamic involvement. Unfortunately, efficient therapeutic options for treatment of sequelae due to hypothalamic syndrome (such as obesity and neuropsychological deficits) are currently not available. Accordingly, further studies aiming at efficient treatment for hypothalamic sequelae in these patients are warranted.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Dr. Qing Liu (Department of Neurosurgery, Xiangya Hospital, Central South University; The Institute of Skull Base Surgery & Neuro-oncology at Hunan; Neurosurgery Institute of the Central South University, Changsha, China) and Assistant Editor Dr. Jian Yuan (Department of Neurosurgery, Xiang Ya Hospital, Changsha, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.10.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Müller HL. Craniopharyngioma. Endocr Rev 2014;35:513-43. [Crossref] [PubMed]
- Larkin SJ, Ansorge O. Pathology and pathogenesis of craniopharyngiomas. Pituitary 2013;16:9-17. [Crossref] [PubMed]
- Erfurth EM, Holmer H, Fjalldal SB. Mortality and morbidity in adult craniopharyngioma. Pituitary 2013;16:46-55. [Crossref] [PubMed]
- Muller HL. Risk-adapted, long-term management in childhood-onset craniopharyngioma. Pituitary 2017;20:267-81. [Crossref] [PubMed]
- Brastianos PK, Shankar GM, Gill CM, et al. Dramatic Response of BRAF V600E Mutant Papillary Craniopharyngioma to Targeted Therapy. J Natl Cancer Inst 2015;108:djv310. [Crossref] [PubMed]
- Brastianos PK, Taylor-Weiner A, Manley PE, et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet 2014;46:161-5. [Crossref] [PubMed]
- Goschzik T, Gessi M, Dreschmann V, et al. Genomic Alterations of Adamantinomatous and Papillary Craniopharyngioma. J Neuropathol Exp Neurol 2017;76:126-34. [PubMed]
- Holsken A, Sill M, Merkle J, et al. Adamantinomatous and papillary craniopharyngiomas are characterized by distinct epigenomic as well as mutational and transcriptomic profiles. Acta Neuropathol Commun 2016;4:20. [Crossref] [PubMed]
- Larkin SJ, Preda V, Karavitaki N, et al. BRAF V600E mutations are characteristic for papillary craniopharyngioma and may coexist with CTNNB1-mutated adamantinomatous craniopharyngioma. Acta Neuropathol 2014;127:927-9. [Crossref] [PubMed]
- Farzin M, Toon CW, Clarkson A, et al. BRAF V600E mutation specific immunohistochemistry with clone VE1 is not reliable in pituitary adenomas. Pathology 2014;46:79-80. [Crossref] [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [Crossref] [PubMed]
- Aylwin SJ, Bodi I, Beaney R. Pronounced response of papillary craniopharyngioma to treatment with vemurafenib, a BRAF inhibitor. Pituitary 2016;19:544-6. [Crossref] [PubMed]
- Gaston-Massuet C, Andoniadou CL, Signore M, et al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proceedings of the National Academy of Sciences of the United States of America 2011;108:11482-7. [Crossref] [PubMed]
- Stache C, Holsken A, Fahlbusch R, et al. Tight junction protein claudin-1 is differentially expressed in craniopharyngioma subtypes and indicates invasive tumor growth. Neuro Oncol 2014;16:256-64. [Crossref] [PubMed]
- Martinez-Barbera JP. 60 YEARS OF NEUROENDOCRINOLOGY: Biology of human craniopharyngioma: lessons from mouse models. J Endocrinol 2015;226:T161-72. [Crossref] [PubMed]
- Andoniadou CL, Matsushima D, Mousavy Gharavy SN, et al. Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 2013;13:433-45. [Crossref] [PubMed]
- Muller HL. Paediatrics: surgical strategy and quality of life in craniopharyngioma. Nat Rev Endocrinol 2013;9:447-9. [Crossref] [PubMed]
- Elowe-Gruau E, Beltrand J, Brauner R, et al. Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. J Clin Endocrinol Metab 2013;98:2376-82. [Crossref] [PubMed]
- Hankinson TC, Palmeri NO, Williams SA, et al. Patterns of care for craniopharyngioma: survey of members of the american association of neurological surgeons. Pediatr Neurosurg 2013;49:131-6. [Crossref] [PubMed]
- Mortini P, Gagliardi F, Boari N, et al. Surgical strategies and modern therapeutic options in the treatment of craniopharyngiomas. Crit Rev Oncol Hematol 2013;88:514-29. [Crossref] [PubMed]
- Hoffmann A, Warmth-Metz M, Gebhardt U, et al. Childhood craniopharyngioma - changes of treatment strategies in the trials KRANIOPHARYNGEOM 2000/2007. Klin Padiatr 2014;226:161-8. [Crossref] [PubMed]
- Puget S, Garnett M, Wray A, et al. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg 2007;106:3-12. [PubMed]
- Müller HL, Gebhardt U, Faldum A, et al. Xanthogranuloma, Rathke's cyst, and childhood craniopharyngioma: results of prospective multinational studies of children and adolescents with rare sellar malformations. J Clin Endocrinol Metab 2012;97:3935-43. [Crossref] [PubMed]
- Müller HL, Gebhardt U, Teske C, et al. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. Eur J Endocrinol 2011;165:17-24. [Crossref] [PubMed]
- Mallucci C, Pizer B, Blair J, et al. Management of craniopharyngioma: the Liverpool experience following the introduction of the CCLG guidelines. Introducing a new risk assessment grading system. Childs Nerv Syst 2012;28:1181-92. [Crossref] [PubMed]
Cite this article as: Peng J, Müller HL. Personalized therapy in craniopharyngioma—novel perspectives and limitations. J Xiangya Med 2017;2:71.


