
Minimally invasive direct coronary artery bypass in a patient with combined severe coronary artery disease and pancreatic ductal adenocarcinoma
Introduction
Patients with coronary artery disease and a malignancy are not uncommon because of increasing life expectancy and improved diagnosis (1). Pancreatic ductal adenocarcinoma has a poor prognosis, and surgical tumor resection is the only curative treatment (2). In cancer patients with severe coronary artery disease involving the left anterior descending (LAD) artery or left main trunk, myocardial revascularization has priority over treatment of cancer (3). The optimal myocardial revascularization procedure for severe coronary artery disease with a concomitant malignancy is controversial. We here report the treatment of a patient with severe coronary artery disease and pancreatic ductal adenocarcinoma.
Case presentation
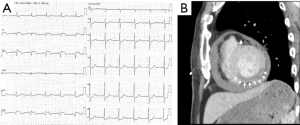
A 74-year-old man was presented in the emergency department with a sudden loss of consciousness. He had a history of diabetes with severe retinopathy and nephropathy, and was under insulin treatment. The patient arrived in a state of coma with a blood pressure of 70/44 mmHg. Abnormal Q waves with ST elevation in II, III, and aVF induction were seen on electrocardiography (Figure 1A). Transthoracic echocardiography (UCG) revealed hyperechoic pericardial effusion. Computed tomography (CT) ruled out acute aortic dissection, but found that the coronary arteries were highly calcified and the inferior wall of the left ventricle was insufficiently enhanced (Figure 1B). The patient was diagnosed with cardiac rupture following inferior myocardial infarction. Emergent coronary revascularization was not performed because of late reperfusion. Pericardiocentesis resolved the shock, and after admitting to the intensive care unit, the patient’s condition gradually improved with medical treatment. About 1 month after hospitalization, the patient’s activity recovered to the level before admission.
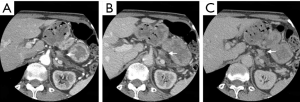
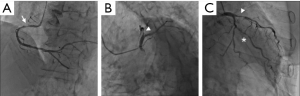
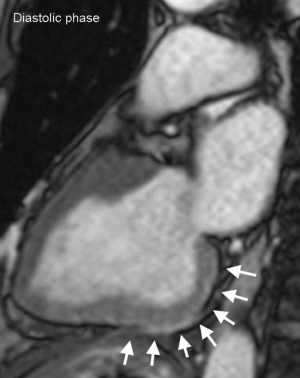
On admission, CT incidentally detected a pancreatic tumor. Subsequent dynamic CT showed a highly atrophic pancreas with a slowly enhancing tumor (Figure 2). The finding was consistent with pancreatic ductal adenocarcinoma, and the preoperative stage was classified as IIA (T3N0M0). Clinical re-evaluation showed severe diabetic nephropathy with a serum creatinine level of 4.5 mg/dL. Coronary artery angiography revealed severe stenosis of the proximal right coronary artery (RCA) and LAD artery (Figure 3A,B) and moderate stenosis of the left circumflex (LCX) artery (Figure 3C). UCG and cardiac magnetic resonance imaging showed aneurysmal morphology of the inferior wall of the left ventricle, suggesting no myocardial viability of the RCA territory (Figure 4). We scheduled minimally invasive direct coronary artery bypass (MIDCAB) for the LAD artery prior to resection of the pancreatic tumor.
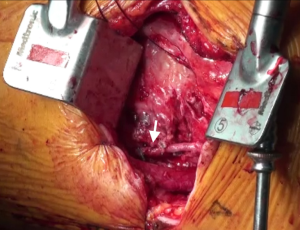
The patient was placed in a 30° right lateral decubitus position. An 8 cm incision was made in the left fourth intercostal space beginning 2 cm from the left border of the sternal bone and extending laterally. After cutting the fourth costal cartilage, a ThoraTrak rib spreader (Medtronic, Minneapolis, Minn) was inserted. The left internal thoracic artery (LITA) was fully harvested under direct vision. Although severe adhesion in the pericardial space interfered with the dissection and confirmation of the LAD artery, the LITA was anastomosed side-to-side to the LAD artery using an 8-0 polypropylene suture (Figure 5).
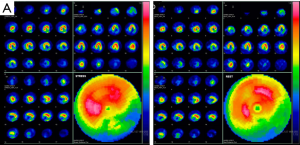
The postoperative course was uneventful. Postoperative single-photon emission CT showed no irreversible ischemia in the LAD territory (Figure 6). The patient was discharged, and then, readmitted for distal pancreatectomy 3 months after the MIDCAB. Postoperative pathological evaluation confirmed a stage IIB pancreatic ductal adenocarcinoma. The patient returned to daily life without hemodialysis.
Discussion
Surgical management of patients with combined coronary artery disease and a malignancy is challenging (1). Pancreatic ductal adenocarcinoma has an extremely poor prognosis with 5-year survival of 5% to 10%, and surgical tumor resection is the only curative therapy (2).
In cancer patients with severe coronary artery disease involving the LAD artery or the left main trunk, myocardial revascularization takes priority over cancer treatment (3). Myocardial revascularization for coronary artery disease with a concomitant malignancy includes percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG). PCI entails the placement of either bare-metal stents (BMS) or drug-eluting stents (DES), both of which require dual antiplatelet therapy (DAPT) to prevent thrombotic complications (4). The placement of BMS is followed by 1 month of DAPT, but in-stent restenosis occurs in 16% to 44% of patients with BMS (5). Although DES are superior to BMS in all types of lesions, 6 to 12 months of DAPT are required after placement (6). Early discontinuation of DAPT may cause myocardial ischemia because of in-stent thrombosis, and non-cardiac surgery with continuation of DAPT has been associated with a high incidence of bleeding complications (7).
Surgery for coronary artery disease and a malignancy can be performed simultaneously as a combined procedure or separately as two-stage procedures. Each approach has its own advantages and disadvantages regarding cost, hospital stay, and tumor treatment (8). In this patient, we considered that the risk was too high to perform combined CABG and distal pancreatectomy because of weakness and frailty. In two-stage procedures, off-pump CABG is an effective myocardial revascularization in patients with combined coronary artery disease and a malignancy (8). However, this patient was predisposed to mediastinitis because of severe diabetes and chronic kidney disease (9). MIDCAB is coronary artery bypass for the LAD artery using the LITA via a left anterior mini-thoracotomy, and has the advantages of rapid recovery, reduced pain, decreased bleeding, and avoidance of mediastinitis (10). A major concern of two-stage operations for patients with combined coronary artery disease and a malignancy is the interval between cardiac surgery and cancer treatment (8). Delay in cancer treatment may result in progression of the malignancy. Therefore, MIDCAB prior resection of the pancreatic tumor was considered to be a reasonable treatment in this case.
Conclusions
We reported the treatment of a patient with severe coronary artery disease and pancreatic ductal adenocarcinoma. Pancreatic ductal adenocarcinoma is a malignancy with an extremely poor prognosis, with surgical tumor resection as the only curative treatment. PCI should be avoided immediately before non-cardiac surgery, because discontinuation of DAPT risks in-stent thrombosis. Two-stage surgery with MIDCAB, followed by tumor resection, is effective and reliable for patients with combined coronary artery disease and a malignancy, especially if the patient is weak and frail.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.07.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Darwazah AK. Surgical management of coronary artery disease associated with malignancy. J Card Surg 2012;27:581-8. [Crossref] [PubMed]
- Åkerberg D, Ansari D, Andersson R. Re-evaluation of classical prognostic factors in resectable ductal adenocarcinoma of the pancreas. World J Gastroenterol 2016;22:6424-33. [Crossref] [PubMed]
- Krone RJ. Managing coronary artery disease in the cancer patient. Prog Cardiovasc Dis 2010;53:149-56. [Crossref] [PubMed]
- Patil RK, Swaminathan RV, Feldman DN. Continuation of Dual-Antiplatelet Therapy Following Percutaneous Revascularization with a Drug-Eluting Stent: What Duration Is Optimal? Curr Atheroscler Rep 2015;17:63. [Crossref] [PubMed]
- Baschet L, Bourguignon S, Marque S, et al. Cost-effectiveness of drug-eluting stents versus bare-metal stents in patients undergoing percutaneous coronary intervention. Open Heart 2016;3:e000445. [Crossref] [PubMed]
- Giustino G, Baber U, Sartori S, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: a systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol 2015;65:1298-310. [Crossref] [PubMed]
- Kałuza GL, Joseph J, Lee JR, et al. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. J Am Coll Cardiol 2000;35:1288-94. [Crossref] [PubMed]
- Darwazah AK, Osman M, Sharabati B. Use of off-pump coronary artery bypass surgery among patients with malignant disease. J Card Surg 2010;25:1-4. [Crossref] [PubMed]
- Ohira S, Doi K, Numata S, et al. Impact of Chronic Kidney Disease on Long-Term Outcome of Coronary Artery Bypass Grafting in Patients With Diabetes Mellitus. Circ J 2016;80:110-7. [Crossref] [PubMed]
- Holzhey DM, Jacobs S, Mochalski M, et al. Seven-year follow-up after minimally invasive direct coronary artery bypass: experience with more than 1300 patients. Ann Thorac Surg 2007;83:108-14. [Crossref] [PubMed]
Cite this article as: Ikeda A, Nishina H, Miyamoto R, Tadano S, Inagawa S, Hiramatsu Y, Jikuya T. Minimally invasive direct coronary artery bypass in a patient with combined severe coronary artery disease and pancreatic ductal adenocarcinoma. J Xiangya Med 2017;2:63.


