Treatment of cancer-associated venous thromboembolism by new oral anticoagulants: a meta-analysis
Introduction
The risk of venous thromboembolism (VTE) is fourfold to sevenfold in patients with malignancy compared to those without (1,2). VTE is the leading cause of mortality in patients with cancer and results in increased morbidity (3,4). It also contributes to an increased cost of care (5). The management of cancer-associated thrombosis poses many challenges due to the simultaneously increased risk of bleeding seen in patients receiving anticoagulation treatment (6,7). Given the delicate hemostatic balance in patients with cancer, the assessment of both thrombosis and bleeding risks is vital for effective management of hematological complications. The 2016 CHEST Guideline and Expert Panel Report advocates the use of low-molecular-weight heparin (LMWH) over a vitamin K antagonist (VKA) for the first three months after the diagnosis of VTE in cancer patients (Grade 2B recommendation) (8). However, a recurrence rate of VTE as high as 15% per year in cancer patients has compelled practitioners to consider indefinite anticoagulation in this population. Therefore, extended anticoagulant therapy is recommended in these patients despite the increased risks of bleeding (8).
The issues concerning the long-term use of LMWH include its feasibility, the cost of the drug, and quality of life. VKA therapy for VTE in cancer patients is suboptimal due to the twofold risk of relapse and the threefold risk of bleeding compared to the corresponding rates for non-cancer patients (9). VKA therapy for VTE in cancer patients also poses challenges in management, which include potential interactions with multiple medications along with associated difficulty in controlling the international normalized ratio (INR), thereby resulting in poor-quality anticoagulation control, as echoed by a reduced time in the therapeutic range (TTR) (10). New direct anti-Xa and anti-IIa oral anticoagulants with no recommended laboratory monitoring requirements, predictable responses, oral administration, and fixed dose regimens are attractive alternatives for the treatment of VTE in cancer patients. However, given the small proportion of cancer patients included in the trials with these newer agents, a meta-analysis specifically including cancer patients from these trials will provide more evidence of their efficacy and safety. Although these trials have proportion of patients with cancer, many of them do not specify the type of malignancy. Lack of this data demands a RCT in specific types of cancers with these new agents.
Prior meta-analyses have assessed the effectiveness of newer oral anticoagulants (NOAs) for the secondary prevention of VTE. Our study also includes trials where NOAs were compared with LMWH for thromboprophylaxis in hospitalized patients, and a proportion of these patients had malignancy. We therefore conducted an updated meta-analysis for efficacy and safety outcomes.
Methods
Search strategy and data sources
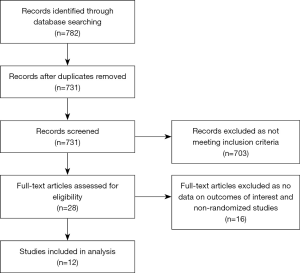
All randomized controlled trials (RCTs) published as of April 30, 2017 were identified after conducting a search using the PubMed, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) databases. Abstracts were retrieved and independently reviewed by two authors, Satyanarayana R. Vaidya and Sonu Gupta, for eligibility. We also searched the bibliographies of the original trials, the meta-analyses, and review articles. The search terms used were as follows: venous thromboembolism, cancer, malignancy, thromboprophylaxis, warfarin, dabigatran, rivaroxaban, apixaban, edoxaban, or oral factor Xa inhibitor, an oral thrombin inhibitor. The meta-analysis was conducted as per the recommendations from the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (11,12). The search algorithm is demonstrated in Figure 1.
Study selection
Studies which met the following inclusion criteria were included.
- Randomized controlled trials
- Trials comparing NOAs with low molecular weight heparin
- Trials comparing NOAs with combination of LMWH or heparin and therapeutic doses of VKAs in patients with VTE,
- Trials which recruited patients with active cancer.
Observational studies, RCTs which did not include cancer patients, studies with no comparator arms were excluded. The outcomes of interest included VTE recurrence rates, major and clinically relevant bleeding.
Data extraction
The data were extracted independently by two authors, Satyanarayana R. Vaidya and Sonu Gupta, from the trials, including the year of publication, study design, sample size, mean age, sex, therapeutic indication, type of drug, dose, duration of follow-up, and number of patients with active cancer. The efficacy outcomes included the number of VTE recurrences; safety outcomes included major or clinically relevant bleeding. The characteristics of the included studies are summarized in Table 1.
Table 1
| Study | Year | Study design | Study medication | Dose of medication | Control | Duration of study (months) | Number of patients randomized | Number of patients with active cancer (%) | Time in therapeutic range (TTR) (%) | Mortality: study medication/control | Recurrent VTE, study medication/control | Clinically relevant bleeding: study medication/control | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agnelli et al. (AMPLIFY) | 2013 | Double blind | Apixaban | 10 mg bid for 7 days followed by 5 mg bid | LMWH/VKA | 6 | 5,395 | 169 (3.1) | 61 | 1.5/1.9 | 2.3/2.7 | 4.3/9.7 | 5 |
| Agnelli et al. (ODIXa-DVT) | 2007 | Double blind | Rivaroxaban | 10 or 20 or 30 mg bid or 40 mg qd | LMWH/VKA | 3 | 528 | 16 (3.0) | 60 | 2.7/0.8 | 1.9 or 2.0 or 1.8 or 2.6 vs. 0.9 |
5.0 or 9.4 or 10.7 or 11.6 vs. 6.3 | 5 |
| Buller et al. (EINSTEIN DVT) | 2008 | Double blind | Rivaroxaban | 20 or 30 or 40 mg q daily | LMWH/VKA | 3 | 543 | 51 (9.4) | 50.3 | 3.0 or 6.0 or 1.5 vs. 3.6 |
2.6 or 3.6 or 1.7 vs. 6.9 |
5.9 or 6.0 or 2.2 vs. 8.8 |
5 |
| Buller et al. (BOTTICELLI DOSE RANGING) | 2008 | Double blind | Apixaban | 5 or 10 mg bid or 20 mg q daily | LMWH/VKA | 3 | 520 | 37 (7.1) | 57 | 2.3 or 0.7 or 0.8 vs. 0 |
2.6 or 3.2 or 1.7 vs. 2.5 |
8.6 or 4.5 or 8.9 vs. 7.9 |
5 |
| Bauersachs et al. (EINSTEIN DVT) | 2010 | Open | Rivaroxaban | 15 mg bid for 3 wks followed by 20 mg q daily | LMWH/VKA | 12 | 3,449 | 207 (6.0) | 57.7 | 2.2/2.9 | 2.1/3.0 | 8.1/8.1 | 5 |
| Buller et al. (HOKUSAI) | 2013 | Double blind | Heparin/edoxaban | 60 mg once daily or 30 mg once daily | Heparin or LMWH/VKA | 12 | 8,240 | 208 (2.5) | 63.5 | 3.2/3.1 | 3.2/3.5 | 8.5/10.3 | 5 |
| Buller et al. (EINSTEIN PE) | 2012 | Open | Rivaroxaban | 15 mg bid for 3 weeks followed by 20 mg qd | LMWH/VKA | 12 | 4,832 | 223 (4.6) | 62.7 | 2.4/2.1 | 2.1/1.8 | 10.3/11.4 | 3 |
| Goldhaber et al. (ADOPT) | 2011 | Double blind | Apixaban | 2.5 mg bid for 30 days | Enoxaparin | 1 | 6,528 | 211 (3.23) | NR | 1.73/1.61 | 2.71/3.06 | 0.47/0.19 | 5 |
| Cohen et al. (MAGELLAN) | 2011 | Double blind | Rivaroxaban | 10 mg q daily | Enoxaparin | 1 | 8,101 | 592 (7.3) | NR | 0.1 vs. 0.2 | 3.0 vs. 3.1 | 2.8 vs. 1.2 | 4 |
| Schulman et al. (RECOVER I) | 2009 | Double blind | Dabigatran | 150 mg bid | Heparin/VKA | 6 | 2,539 | 121 (4.8) | 60 | 1.6 vs. 1.7 | 2.7 vs. 2.5 | 5.6 vs. 8.8 | 5 |
| Schulman et al. (RECOVER II) | 2014 | Double blind | Dabigatran | 150 mg bid | Heparin/VKA | 6 | 2,589 | 100 (3.9) | 57 | 2.0 vs. 1.9 | 2.3 vs. 2.2 | 5.0 vs. 7.9 | 5 |
| Schulman et al. (REMEDY) | 2013 | Double blind | Dabigatran | 150 mg bid | Heparin/VKA | 6–36 | 2,586 | 119 (4.2) | 65.3 | 1.2 vs. 1.3 | 1.8 vs. 1.3 | 5.6 vs. 10.2 | 5 |
VKA, Vitamin K antagonist; LMWH, low molecular weight heparin.
Quality assessment
The quality of the studies was assessed by using the Jadad scale (13). A score of ≥3 was considered to define a high-quality study, with all twelve studies having this score.
Statistical analysis
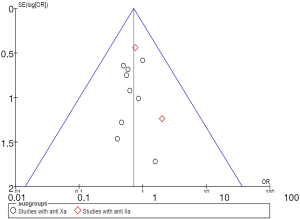
The statistical analysis was conducted in line with the recommendations of the Cochrane Collaboration and PRISMA guidelines using Review Manager (RevMan) version 5.3 software (Cochrane, Oxford, UK). The random effects model of DerSimonian and Laird was used because of the clinical and methodological heterogeneity of the studies. The relative risk ratios (RRs) and 95% confidence intervals (CIs) were calculated using the inverse variance method. Heterogeneity was considered as the proportion of the total variation observed between the trials, which was due to the differences between the trials rather than a sampling error, and was assessed using the I2 statistic. An I2<25% was considered to represent low heterogeneity, and an I2>75% was considered to represent high heterogeneity (14). The publication bias was estimated visually through the use of funnel plots (Figure 2). Inspection of the funnel plot for the outcomes for VTE did not reveal any publication bias.
Results
Our primary search yielded 782 studies. Twelve studies fulfilled our inclusion criteria and were included in the meta-analysis (15-26). These twelve studies had included active cancer patients and reported their outcomes. Of the twelve trials, five studied rivaroxaban (16,18-20,23), three studied apixaban (15,17,22), three studied dabigatran (24-26), and one studied edoxaban (21). Of the twelve studies, ten compared NOAs with standard treatment of VTE (LMWH &VKA). Two studies compared NOAs with LMWH only in acutely ill hospitalized patients. In aggregate, all the above studies included a total of 2,054 active cancer patients. The duration of follow-up ranged from one to 36 months. The study sizes ranged from 520 to 8,240 patients, with the rates of included cancer patients ranging from 2.5–9.4%.
Recurrence of VTE
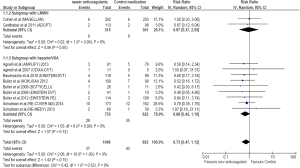
Twenty-nine of 733 cancer patients treated with NOAs developed VTE compared to 35 of the 622 patients treated with standard treatment. Compared to standard treatment, NOAs showed a non-significant reduction in VTE [RR =0.68; 95% CI =0.42–1.10; P=0.12] (Figure 3). There was no heterogeneity for this outcome.
Bleeding events
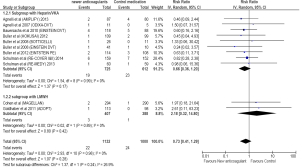
Nineteen of 725 cancer patients treated with NOAs developed major bleeding compared to 23 of the 612 patients treated with standard treatment. Compared to standard treatment, NOAs showed a non-significant reduction in major bleeding (RR =0.66; 95% CI =0.36–1.20; P=0.17) (Figure 4).
Non-major clinically relevant bleeding occurred in 93 of 725 cancer patients treated with NOAs compared to 95 of 612 cancer patients treated with standard treatment. Compared to standard treatment, a non-significant reduction in clinically relevant bleeding was shown with the use of NOAs (RR =0.85; 95% CI =0.65–1.10; P=0.22) (Figure 5).
Subgroup analysis
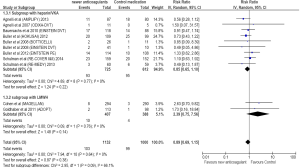
A subgroup analysis of trials comparing anti-Xa and anti-IIa with control medications was conducted. Of the twelve trials, nine studies with anti-Xa and three with anti-IIa were analyzed. The RR for recurrent VTE showed rates of 0.67 (95% CI =0.40–1.14) for anti-Xa and 0.86 (95% CI =0.40–1.85) for anti-IIa, respectively.
NOAs compared to LMWH only in hospitalized cancer patients
A subgroup analysis of studies showed that NOAs were comparable to LMWH in prevention of VTE in acutely ill hospitalized cancer patients. (RR =0.97; 95% CI =0.37–2.55; P=0.95). Higher major bleeding (RR =2.18; 95% CI =0.32–14.80; P=0.42) and clinically relevant bleeding (RR =2.39; 95% CI =0.75–7.56; P=0.14) rates were observed with NOAs compared to LMWH in acutely ill hospitalized cancer patients. However, the results did not reach statistical significance.
Discussion
The results of this meta-analysis of randomized trials comparing the efficacy and safety of NOAs with standard VTE treatment (heparin & VKA combination) in cancer patients suggest that NOAs are comparable in their efficacy and safety to standard treatment for VTE in cancer patients with a non-significant lower event rate. The mild reduction of both VTE and bleeding events noted was consistent across all studies. However, NOAs tend to show higher rates of bleeding compared to LMWH only in acutely ill hospitalized cancer patients.
The efficacy of NOAs in preventing the recurrence of VTE found in our study is similar to that found in a prior meta-analysis conducted by Vedovati et al. (27), where VTE recurred in 3.9% of cancer patients treated with NOAs as compared to 6.0% of those treated conventionally. In the randomized trial “Low Molecular Weight Heparin versus Oral Anticoagulant therapy for the prevention of recurrent Venous Thromboembolism in patients with cancer (CLOT) study”, recurrent VTE was found in 8% of patients in randomized treatment with dalteparin compared to 16% in those with oral anticoagulation treatment, which was equivalent to a 50% risk reduction. In this study, the level of anticoagulation as estimated by time in TTR was around 46%. Compared to this value, the mean TTR of conventional treatment in randomized trials with NOAs is around 59.45%. This increased intensity of anticoagulation in the conventional treatment group in randomized trials with NOAs compared to those in the CLOT study might explain the lower risk reduction of 27% found in our study.
In our analysis, the bleeding rates with NOAs were comparable to those with heparin &VKA combination in cancer patients. In a subgroup analysis of acutely ill hospitalized cancer patients, where NOAs were compared to LMWH for prevention of VTE, both major and clinically relevant bleeding rates in the NOA cohort were higher than corresponding rates among the LMWH treated group. However, in this subgroup, acutely-ill hospitalized medical patients were included, which was different from the relatively healthier patients in the randomized trials with NOAs. In a study by Decousus et al. on acutely ill medical patients, active gastroduodenal ulceration, prior bleeding, low platelet count, advanced age, hepatic or renal failure, the presence of a central venous catheter, rheumatic disease, and cancer were identified as risk factors upon admission and were associated with in-hospital bleeding (28). Furthermore, the median age of patients in the MAGELLAN trial (23) was around 71 years, and around 21% of patients had impaired renal function (creatinine clearance of less than 50 mL per minute), and about 7% of the patients had active cancer. Additionally, 4% had acute inflammatory or rheumatic disease. These above risk factors present at admission in these patients could have contributed to the increased rates of bleeding observed with extended thromboprophylaxis.
The inclusion of data from only randomized trials strengthens our analysis. There was no significant heterogeneity observed for all the outcomes, suggesting that the results were consistent across all studies. Conversely, our study has limitations inherent to any meta-analysis. The datasets included in our analysis were not matched for type and stage of cancer or type of VTE. Most of the studies included in the meta-analysis excluded patients with anemia, high bleed risk, thrombocytopenia, and renal failure. Therefore, the results of this study are applicable to select group of patients without the above parameters. Unfortunately, a precise definition of active cancer was not available in the various studies. These limitations support the need for ad hoc clinical trials with NOAs evaluating efficacy and safety exclusively among cancer patients.
Conclusions
NOAs seem to be comparable in efficacy and safety to conventional treatment in prevention of VTE recurrence among cancer patients. However, increased rates of bleeding were observed with their use as compared to LMWH use in acutely-ill hospitalized patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.07.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Blom JW, Doggen CJ, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. Jama 2005;293:715-22. [Crossref] [PubMed]
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809-15. [Crossref] [PubMed]
- Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost 2007;5:632-4. [Crossref] [PubMed]
- Gussoni G, Frasson S, La Regina M, et al. Three-month mortality rate and clinical predictors in patients with venous thromboembolism and cancer. Findings from the RIETE registry. Thromb Res 2013;131:24-30. [Crossref] [PubMed]
- Khorana AA, Dalal MR, Lin J, et al. Health care costs associated with venous thromboembolism in selected high-risk ambulatory patients with solid tumors undergoing chemotherapy in the United States. Clinicoecon Outcomes Res 2013;5:101-8. [Crossref] [PubMed]
- Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002;100:3484-8. [Crossref] [PubMed]
- Monreal M, Falga C, Valdes M, et al. Fatal pulmonary embolism and fatal bleeding in cancer patients with venous thromboembolism: findings from the RIETE registry. J Thromb Haemost 2006;4:1950-6. [Crossref] [PubMed]
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 2016;149:315-52. [Crossref] [PubMed]
- Coleman R, MacCallum P. Treatment and secondary prevention of venous thromboembolism in cancer. Br J Cancer 2010;102:S17-23. [Crossref] [PubMed]
- De Caterina R, Husted S, Wallentin L, et al. Vitamin K antagonists in heart disease: current status and perspectives (Section III). Position paper of the ESC Working Group on Thrombosis--Task Force on Anticoagulants in Heart Disease. Thromb Haemost 2013;110:1087-107. [Crossref] [PubMed]
- Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999;354:1896-900. [Crossref] [PubMed]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [Crossref] [PubMed]
- Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Bmj 2003;327:557-60. [Crossref] [PubMed]
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013;369:799-808. [Crossref] [PubMed]
- Agnelli G, Gallus A, Goldhaber SZ, et al. Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT (Oral Direct Factor Xa Inhibitor BAY 59-7939 in Patients With Acute Symptomatic Deep-Vein Thrombosis) study. Circulation 2007;116:180-7. [Crossref] [PubMed]
- Buller H, Deitchman D, Prins M, et al. Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging study. J Thromb Haemost 2008;6:1313-8. [Crossref] [PubMed]
- Buller HR, Lensing AW, Prins MH, et al. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT Dose-Ranging Study. Blood 2008;112:2242-7. [Crossref] [PubMed]
- Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499-510. [Crossref] [PubMed]
- Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012;366:1287-97. [Crossref] [PubMed]
- Büller HR, Decousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013;369:1406-15. [Crossref] [PubMed]
- Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med 2011;365:2167-77. [Crossref] [PubMed]
- Cohen AT, Spiro TE, Buller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513-23. [Crossref] [PubMed]
- Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009;361:2342-52. [Crossref] [PubMed]
- Schulman S, Kearon C, Kakkar AK, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med 2013;368:709-18. [Crossref] [PubMed]
- Schulman S, Kakkar AK, Goldhaber SZ, et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014;129:764-72. [Crossref] [PubMed]
- Vedovati MC, Germini F, Agnelli G, et al. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest 2015;147:475-83. [Crossref] [PubMed]
- Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest 2011;139:69-79. [Crossref] [PubMed]
Cite this article as: Vaidya SR, Gupta S, Devarapally SR. Treatment of cancer-associated venous thromboembolism by new oral anticoagulants: a meta-analysis. J Xiangya Med 2017;2:55.