Elevated D-dimer in patient with acute aortic dissection
Introduction
Acute aortic dissection is an uncommon but severe disease with high mortality rates (1). About 40% of patients remained undiagnosed until necropsy (2) for patient presenting with acute chest or acute back pain, some clinical features may be effective in identifying acute aortic dissection: tearing chest pain, aortic enlargement on chest X-ray and blood pressure (or arm pulse) difference most than 20 mmHg (3). Electrocardiogram and chest X-ray must be obtained for the evaluation but are limited for ruling-out aortic dissection (4). Imaging with CT angiography, transesophageal echocardiogram or magnetic resonance angiography recommended to confirm or exclude diagnosis (5,6).
Case presentation
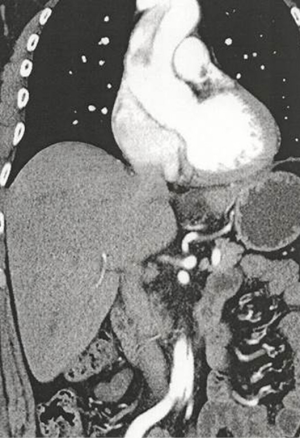
The prehospital emergency team was dispatched at 7:30 pm to a 54-year-old man with chest pain. The pain began gradually at 2:00 pm after he lifted a heavy cement bag, and was described as ‘constrictive’ without any radiation. He described a history of abdominal pain for last six months, but a workup including blood tests and echocardiographic imaging was negative. His past medical history was negative for hypertension, but high level of glycemia and cholesterol had previously been noted without treatment. He was a current smoker and his BMI was 28.98 kg/m2. There were no other risk factors for aortic dissection. Physical examination was normal, without signs of heart failure or pulmonary embolism (Geneva score =0), pulses were symmetric, with bilateral blood pressure of 13/8, no fever, no neurologic deficit. Electrocardiogram (done at 07:45 pm) showed flat T-waves in D2-D3-VF without STEMI. An acute coronary syndrome was initially suspect, the patient received aspirin and was send to the emergency department for further investigations. In the ED (arrival at 08:35 pm), troponin was negative but D-dimer testing was most than 4,000 ng/mL (results obtained at 10:00 pm). Transthoracic echocardiography showed normal left and right cardiac function and no evidence of aortic regurgitation. However, the cardiologist suspected an abdominal aortic flap. CT angiography of the entire aorta was performed and identified a type-A (Stanford) aortic dissection, extending from the supra-coronary ascending aorta to the left iliac artery with complete thrombosis of the false lumen (Figure 1). Successful surgery (valve sparing with remodelling and external aortic annuloplasty and hemiarch replacement) was performed at midnight, without complications during follow-up. He was discharged from the hospital after a week.
Discussion
Our case report highlights two important points. First, the common scenario of initial misdiagnosis of patient with acute aortic dissection. Our patient did not present with sudden chest pain, and did not have a history of blood pressure. His chest X-ray was normal, and characteristics of the chest pain and subtle changes on the electrocardiogram initially suggested an acute coronary syndrome. Based on the absence of the features, the patient was classified as low risk for aortic dissection. Delays in diagnosis are associated with increased mortality and morbidity, particularly for patients with type-A dissection and partial thrombosis (7). Second, the use of D-dimer as a biomarker may help triage patients with chest pain. D-dimer is a fibrin fragment commonly used for diagnosis of pulmonary embolism with a cut-off level of 500 ng/mL. Meta-analysis reported elevated D-dimer in acute aortic dissection with the same cut-off defined as the threshold for a positive test, with sensibility from 95% to 100%, negative predictive value about 95%, and specificity ranging from 40% to 65% (8-10). Age adjusted cut-offs are recommended using “age × 10 ng/mL” after 50 years old (11). Further studies suggested that D-dimer would be useful as a tool to rule out aortic dissection (8-12). However, the IRAD-Bio investigators identified a need for further studies to clarify the decision tree and how to integrate D-dimer testing in patient with suspected aortic dissection (8). Some studies have been inconclusive because of the cut-off values employed, negative results in patients younger than 70 or patients presenting with complete thrombosis of the false lumen or delayed presentation. These studies have identified a negative correlation between D-dimer and the time from onset symptoms. D-dimer is a predictor of morbidity, including mesenteric ischemia, myocardial infarction, shock or hypotension. However, an elevated D-dimer rate does not predict patient survival (11). Fournier et al. suggested the use of D-dimer for patients with intermediate risk (acute aortic dissection incidence about 30%), and if positive to proceed with imaging if D-dimer is positive. In patient with negative D-dimes the authors suggest to consider alternate diagnosis (4). Gorla et al. described D-dimer as useful for acute aortic communicating dissection and intramural hematoma, with higher rate for the former (11). In the emergency ward, D-dimer as point-of-care tests would be helpful but rarely used in practice. In our case we waited more than 1 h for the results.
D-dimer testing is certainly useful for all patients with chest-pain. This biomarker has a role for screening, a negative test can rule out severe pulmonary embolism and may be useful for patients with low or intermediate likelihood of aortic dissection (9,13). Appropriate imaging is actually always required for definite diagnosis.
In conclusion, 2014 guidelines on thoracic aortic disease recommended testing D-dimers in any patient with suspected acute aortic disease. A positive D-dimer is associated with an increased suspicion of acute aortic disease (14).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.05.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Weber T, Högler S, Auer J, et al. D-dimer in acute aortic dissection. Chest 2003;123:1375-8. [Crossref] [PubMed]
- Eggebrecht H, Naber CK, Bruch C, et al. Value of plasma fibrin D-dimers for detection of acute aortic dissection. J Am Coll Cardiol 2004;44:804-9. [Crossref] [PubMed]
- Siegal EM. Acute aortic dissection. J Hosp Med 2006;1:94-105. [Crossref] [PubMed]
- Fournier Y, Moix PA, Hugli O. Acute aortic dissection: diagnostic usefulness of D-dimer. Rev Med Suisse 2008;4:1759-63. [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol 2010;55:e27-e129. [Crossref] [PubMed]
- Evangelista A, Flachskampf FA, Erbel R, et al. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur J Echocardiogr 2010;11:645-58. [Crossref] [PubMed]
- Tsai TT, Evangelista A, Nienaber CA, et al. Partial thrombosis of the false lumen in patients with acute type B aortic dissection. N Engl J Med 2007;357:349-59. [Crossref] [PubMed]
- Suzuki T, Distante A, Zizza A, et al. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation 2009;119:2702-7. [Crossref] [PubMed]
- Marill KA. Serum D-dimer is a sensitive test for the detection of acute aortic dissection: a pooled meta-analysis. J Emerg Med 2008;34:367-76. [Crossref] [PubMed]
- Shimony A, Filion KB, Mottillo S, et al. Meta-analysis of usefulness of d-dimer to diagnose acute aortic dissection. Am J Cardiol 2011;107:1227-34. [Crossref] [PubMed]
- Gorla R, Erbel R, Kahlert P, et al. Accuracy of a diagnostic strategy combining aortic dissection detection risk score and D-dimer levels in patients with suspected acute aortic syndrome. Eur Heart J Acute Cardiovasc Care 2015; [Epub ahead of print]. [Crossref] [PubMed]
- Sodeck G, Domanovits H, Schillinger M, et al. D-dimer in ruling out acute aortic dissection: a systematic review and prospective cohort study. Eur Heart J 2007;28:3067-75. [Crossref] [PubMed]
- Giannitsis E, Mair J, Christersson C, et al. How to use D-dimer in acute cardiovascular care. Eur Heart J Acute Cardiovasc Care 2017;6:69-80. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [PubMed]
Cite this article as: Goddet NS, Fayard M, Bouchot O, Bete S, Decrouy N, Corege D. Elevated D-dimer in patient with acute aortic dissection. J Xiangya Med 2017;2:45.