
Autonomic nerve preserving in laparoscopic total mesorectal excision
Introduction
The adoption of total mesenteric excision (TME) and combined modality therapy has dramatically improved the oncologic outcome for the patients with rectal cancer during the last 3 decades (1,2). Nonetheless, the improved survival was constantly in company with high incidence of organ dysfunction which might severely compromise quality of life (3,4). In addition to the anterior rectal syndrome, urogenital dysfunctions due to intraoperative inadvertent pelvic autonomic nerve damage are well-recognized complications after rectal cancer surgery (5). The incidence of urinary dysfunction including difficulty emptying the bladder and urinary incontinence has been reported from 0% to 35% (4,6). Male sexual dysfunction includes erectile and ejaculatory problems may reach as high as 79.8% and 72.2%, respectively. For females, sexual dysfunction in relation to dyspareunia, difficulty to achieve orgasm and insufficient vaginal lubrication, is plagued but poorly defined and likely neglected in clinical practice (7,8).
Pelvic autonomic nerve preservation under direct visualization and sharp dissection may be limited and challenging by the anatomical constrains of the curved narrow pelvis, especially for the android pelvis with hypertrophic mesorectum. Laparoscopic technology with the advantages of direct illumination and magnification may enable better visualization and thereby hypothetically help to improve pelvic autonomic nerve preservation. As in previous studies, two multicenter randomized control trails comparing laparoscopic with open surgery have reported worse sexual function in laparoscopic surgery (4,9). While, the later studies showed no differences in outcomes or even in favor of laparoscopic to compare with open surgery (10,11). The increased experience with laparoscopic and understanding of the pelvic nerve anatomy may relate to the improving functional outcomes. A trend towards fewer incidences of urogenital dysfunctions is noticeable with the practice of pelvic autonomic nerve preservation adherence to the TME principles (5,10,12,13).
We believe that favorable urinary and sexual function could be achieved with enhanced vision, adequate traction and countertraction during the laparoscopic TME. Here, we discuss the current understanding of anatomy, key zones at risk and tips and tricks of nerve preservation during the laparoscopic TME (Figure 1).

Pelvic autonomic nerve anatomy
Superior hypogastric plexus (SHP) and hypogastric nerves (HN)
The SHP is a network of pre- and post-ganglionic nerves overlying the abdominal aorta. The plexus is located anterior of the fifth lumbar vertebrae, starts underneath of the inferior mesenteric artery (IMA), run over the sacral promontory and then bifurcates into the right and left HN (15,16). The paired HN run about 2 cm medially to the ureter, move obliquely along the posterolateral wall of the pelvis and finally end as afferent fibres of inferior hypogastric plexus (IHP). The SHP comprises sympathetic nerves, which is originated from the sympathetic trunks alongside the tenth thoracic to the third lumbar vertebrae. Injury to the SHP or HN may result in troubles of ejaculation, decreased orgasm and urinary incontinence (16).
IHP and pelvic splanchnic nerves (PSN)
Besides the sympathetic fibres from the HN, the IHP also receives pelvic parasympathetic fibres from the PSN. The splanchnic nerves arising from the ventral roots of S2–S5 enter the pelvis through sacral foramina, running immediately ventral to the piriform muscles, together with the HN forming the IHP that lies laterally on the pelvic wall at the level of the lower third of rectum. Some branches of the splanchnic nerves run medially to the rectum via the so called “lateral ligaments” and make up the medial segment of the IHP. The lateral ligaments are thought to be condensation of fascia on the dorsolateral side of rectum and connect the rectum to the pelvic parietal fascia. Some suggest the ligaments do not exist and the small, inconstant and frequently unilateral middle rectal artery cross the mesorectum independent of any structure (16-18). The anterior portion of the IHP innervates the bladder and sexual organs. In women, fibers from the IHP travel through underneath the intersection of the ureter and uterine artery to the vesicovaginal and rectovaginal septum forming branches to the bladder, the vagina and the uterus (19). In men, these run in the neurovascular bundles (NVB) incline anteriorly at the lateral corners of the seminal vesicles in the 2 o’clock and 10 o’clock direction. These nerves run laterally outside the Denonvillier’s fascia and continue on the periprostatic plexus supplying branches to the prostate, seminal vesicles, cavernous bodies, and the vas deferens (16,20).
Key zones at risk
Origin of the IMA
The SHP is vulnerable to intercepted during high ligation of the IMA which is intended to achieve complete removal of regional lymph nodes. The SHP fibres lie in front of the aorta and are located in a wide area of dissection. The preaortic plexus injured if the IMA pedicle is ligated flush with the aorta (20). The left trunk of the SHP travels along the left side of the aorta and is very close to the IMA at its origin. So mass clamping of the IMA may increase the risk of damage to the left trunk of the plexus.
Posterior rectum
Although there is still controversy about the facial structures (21), posterior dissection of the rectum should carried out at the avascular plane of loose areolar tissue between the fascia propria anteriorly and the parietal fascia containing the HN posteriorly. A further loose areolar tissue layer could be easily created posterior to the HN. If dissection is too posterior and the HN appears to run into the mesorectum, both the HN and the presacral vein are at risk of damaging. The major risk is to enter the wrong plane at the transition of the mesosigmoid to the mesorectum or not following the correct plane during dissection.
Lateral rectum
At the high level lateral dissection of the mesorectum, the HN is vulnerable to injury if dissection starts or opens the laterorectal reflection of the peritoneum too laterally. During the low lateral dissection, the IHP is vulnerable to injury. The so called “lateral ligament” is a dense connective tissue between rectum and pelvic parietal fascia. The IHP is rarely seen in patients with high body mass index and bulky and fat mesorectum. Injury can occur if the lateral ligament bleeding is not control and blunt dissection is used or excessive medical traction and non-anatomical dissection.
Anterior rectum
There is a very narrow space between the mesorectum and the seminal vesicles and prostate or vaginal. The Denonvillier’s fascia is displaced anterior to the mesorectum and posterior to the seminal vesicles and prostate or vaginal. The NVB lie laterally anterior to the Denonvillier’s fascia and then go down to the urethra at the apex of the prostate. Excessive traction on the rectum “moves” the NVB in the dissection field on the lateral edges. Blind mass clamping if bleeding is not controlled contributes the damage of the NVB. There is no consensus concerning the proper anterior anatomical plane but if the tumor is anterior, the dissection should carry out anterior to the Denonvillier’s fascia, which presents a high risk of nerve damage.
Tips and tricks of nerve preservation in laparoscopic total mesorectal excision
In laparoscopic total mesorectal excision there are a few key zones at risk of nerve injury as mentioned above. To ensure the preservation of pelvic autonomic nerve, a proper surgical technique with step-by-step checklist at the critical points should be carried on without compromise of oncological outcome.
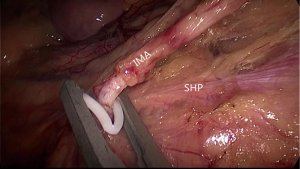
Laparoscopic TME usually starts with a peritoneal incision at the surface of the sacral promontory, dissection from medial to lateral and extend up underneath the duodenum, and then meet the first key area for injury of the IHP. The key of nerve preservation is finding the plane by maintaining sufficient tension. With the help of the assistant lifting the pedicle of the IMA, dissection of the posterior of the IMA could be easy. Under the magnified laparoscopic view, the SHP is visualized covered with ventral fascia. By opening the artery sheath and ligating the pedicle at a distance of 1.5–2 cm from the aorta, the SHP fibres lying in front of the aorta are preserved. Avoiding mass clamping of the IMA and preserving the Gerona’s fascia is needed for the left trunk of the SHP running along the left side of the aorta (Figure 2).
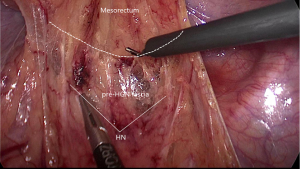
After the dissection of the left Toldt’s space and complete the mobilization of the sigmoid and descend colon, the transition from mesosigmoid to mesorectum is the second zone of iatrogenic nerve injury for the HN. Kinugasa suggest the pre- hypogastric nerves (pre-HGN) fascia anterior to the HN is evident based on historical examination in the retrorectal multilaminar structure. Posterior dissection should be performed at the loose areolar layer between the rectal proper fascia and the pre-HGN fascia (Figure 3). The superior rectal artery is situated just anterior to the rectal propria fascia, which could be used as a landmark to find the plane. Surgeons should follow the plane but not create a new plane, use left hand to counter-traction to keep sufficient tension and keep fascia of rectum clear from other tissue (Figure 4).
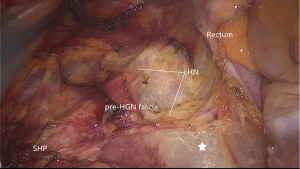
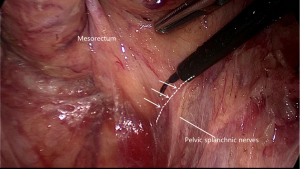
Going all the way down to the level of the forth sacral vertebral will meet the so-called rectosacral fascia which was thought to be a surgical artifact in Kinugasa’s study (21). A sharp incision though this plane will be another loose areolar layer without any risk of nerve damage. The lateral dissection is the third risk zone for injury of the HN and IHP. The lateral dissection is done after the posterior midline dissection has gone as far down to the anorectal junction as possible. Lateral dissection moves from the posterior midline to the left and right posterolateral pelvic wall (Figure 5). Some authors suggest under the illumination and magnification of the laparoscopic technology, the autonomic nerves can serve as the landmark and develop a novel concept of nerve-oriented mesorectal excision. However, The IHP is covered by parietal fascia and rarely seen in obese patients (22). Dissection along the fascia propria may be more appropriate (Figure 6). Identification of the site where fascia of rectum and IHP merge together is needed. Typically, there is a triangle space lying below rectal fascia and nerve plexus. Extending laterally along the line through the triangular space, the IHP attaching to rectal fascia is effectively shrunk. Dissection need be cautious not to break into the mesorectum or the nerve plexus and always keep good tension and small bite. Using the tips mentioned above, the adherent nerves can be slightly teased and peeled off the rectal fascia. After open the laterorectal reflection of the pararectal peritoneum, dissection to the supra-levator space and anterior plane is one of the most vulnerable parts of mesorectum and nerve plexus. Mesorectum stretching out laterally like a coconut rather than cylinder (Figure 7). In order to avoid leftover of mesorectum, dissection should always follow the arc of pelvic floor from posterior to lateral and from known to unknown. After fully mobilization, a clear coconut-like structure can be seen and mesorectum is completely removed.
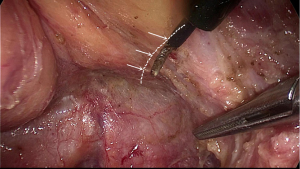
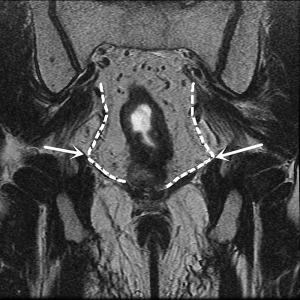
After complete mobilization of the supra-levator space and lateral part of mesorectum, peritoneum is incised about 0.5 cm anterior to the peritoneal reflection. Here we enter the space anterior to Denonvillier’s fascia and meet a new risk area of autonomic never injury. Although Heald suggest the Denonvillier’s fascia forms the anterior surface of the mesorectum, most opinions recommend dissecting in front of Denonvillier’s fascia only when the tumor located anteriorly or there is a risk of compromised circumferential margin. For posterior and lateral tumors, our experience is to transect the Denonvillier’s fascia at the base of seminal vesicle, then dissection is along the propria fascia. Fully mobilization of posterior and lateral mesorectum greatly facilitates the preservation of NVB. Dissection just follows the groove and uses the hook parallel but not vertically to the rectal fascia. In some cases, it looks like there is a shining envelope containing the NVB when it is well preserved (Figure 8).
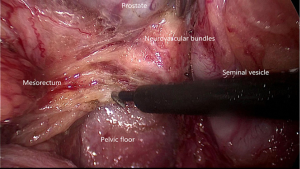
The adoption of TME and combined modality therapy has improved the oncologic outcome greatly in the patients with rectal cancer. Urogenital dysfunctions due to intraoperative inadvertent pelvic autonomic nerve damage are well-recognized. Surgeons should be familiar with the pelvic autonomic nerve anatomy especially the key zones at risk and take advantage of laparoscopic technique in order to improve the functional outcomes of TME.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.04.03). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479-82. [Crossref] [PubMed]
- Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2015;16:979-89. [Crossref] [PubMed]
- Bryant CL, Lunniss PJ, Knowles CH, et al. Anterior resection syndrome. Lancet Oncol 2012;13:e403-8. [Crossref] [PubMed]
- Jayne DG, Brown JM, Thorpe H, et al. Bladder and sexual function following resection for rectal cancer in a randomized clinical trial of laparoscopic versus open technique. Br J Surg 2005;92:1124-32. [Crossref] [PubMed]
- Lange MM, van de Velde CJ. Urinary and sexual dysfunction after rectal cancer treatment. Nat Rev Urol 2011;8:51-7. [Crossref] [PubMed]
- Luca F, Valvo M, Ghezzi TL, et al. Impact of robotic surgery on sexual and urinary functions after fully robotic nerve-sparing total mesorectal excision for rectal cancer. Ann Surg 2013;257:672-8. [Crossref] [PubMed]
- Tekkis PP, Cornish JA, Remzi FH, et al. Measuring sexual and urinary outcomes in women after rectal cancer excision. Dis Colon Rectum 2009;52:46-54. [Crossref] [PubMed]
- Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther 2005;31:1-20. [Crossref] [PubMed]
- Quah HM, Jayne DG, Eu KW, et al. Bladder and sexual dysfunction following laparoscopically assisted and conventional open mesorectal resection for cancer. Br J Surg 2002;89:1551-6. [Crossref] [PubMed]
- Andersson J, Abis G, Gellerstedt M, et al. Patient-reported genitourinary dysfunction after laparoscopic and open rectal cancer surgery in a randomized trial (COLOR II). Br J Surg 2014;101:1272-9. [Crossref] [PubMed]
- McGlone ER, Khan O, Flashman K, et al. Urogenital function following laparoscopic and open rectal cancer resection: a comparative study. Surg Endosc 2012;26:2559-65. [Crossref] [PubMed]
- Shirouzu K, Ogata Y, Araki Y. Oncologic and functional results of total mesorectal excision and autonomic nerve-preserving operation for advanced lower rectal cancer. Dis Colon Rectum 2004;47:1442-7. [Crossref] [PubMed]
- Junginger T, Kneist W, Heintz A. Influence of identification and preservation of pelvic autonomic nerves in rectal cancer surgery on bladder dysfunction after total mesorectal excision. Dis Colon Rectum 2003;46:621-8. [Crossref] [PubMed]
- Tang JH, Ding PR. Video of autonomic nerve preserving in laparoscopic total mesorectal excision. Asvide 2017;4:207. Available online: http://www.asvide.com/articles/1517
- Lu S, Xu Y, Chang S, et al. Clinical anatomy study of autonomic nerve with respective to the anterior approach lumbar surgery. Surg Radiol Anat 2009;31:425-30. [Crossref] [PubMed]
- Moszkowicz D, Alsaid B, Bessede T, et al. Where does pelvic nerve injury occur during rectal surgery for cancer? Colorectal Dis 2011;13:1326-34. [Crossref] [PubMed]
- Jones OM, Smeulders N, Wiseman O, et al. Lateral ligaments of the rectum: an anatomical study. Br J Surg 1999;86:487-9. [Crossref] [PubMed]
- Pak-art R, Tansatit T, Mingmalairaks C, et al. The location and contents of the lateral ligaments of the rectum: a study in human soft cadavers. Dis Colon Rectum 2005;48:1941-4. [Crossref] [PubMed]
- Mauroy B, Demondion X, Bizet B, et al. The female inferior hypogastric (= pelvic) plexus: anatomical and radiological description of the plexus and its afferences--applications to pelvic surgery. Surg Radiol Anat 2007;29:55-66. [Crossref] [PubMed]
- Nagpal K, Bennett N. Colorectal surgery and its impact on male sexual function. Curr Urol Rep 2013;14:279-84. [Crossref] [PubMed]
- Kinugasa Y, Murakami G, Suzuki D, et al. Histological identification of fascial structures posterolateral to the rectum. Br J Surg 2007;94:620-6. [Crossref] [PubMed]
- Runkel N, Reiser H. Nerve-oriented mesorectal excision (NOME): autonomic nerves as landmarks for laparoscopic rectal resection. Int J Colorectal Dis 2013;28:1367-75. [Crossref] [PubMed]
Cite this article as: Tang JH, Ding PR. Autonomic nerve preserving in laparoscopic total mesorectal excision. J Xiangya Med 2017;2:43.


