
Dp71 as a tumor suppressor: combined efforts of nuclear lamin B1 and emerin?
Being one of the largest pathogenic genes, the mutation of dystrophin results in Duchenne muscular dystrophy (DMD). Through alternative usages of internal promoters, Dp71 is a short isoform termed in accordance with its molecular mass, via undergoing alternative splicing of exons 71 and/or 78, Dp71 generate Dp71f (−71/−78) and Dp71d (−71/+78), the most studied isoforms. In Professor Bulmaro Cisneros original discovery, Dp71 was proved to take part in ion and water homeostasis, cell signaling, cell adhesion, and nuclear architecture, reviewed in (1).
Identified as a component of the mitotic spindle and cytokinesis multi-protein apparatuses in PC12 cells, Dp71 was also proved to modulate its cell division cycle. Cancer is a broad group of diseases in which cells divide and grow uncontrollably. The mitotic location of Dp71 prompted us on our initial research of Dp71 in cancer. Unexpectedly, our immunohistochemistry and western blot identified an astonishing reduction of Dp71 expression in both clinical samples from gastric cancer patients and different gastric cancer cell lines. In the clinical sample and cellular research, a ubiquitous Dp71 antibody was used because lack of epitope specific antibody. Our results showed that both Dp71d and Dp71f decreased their expression to the same extent in the gastric cancer. Dp71’s reduction was proved to be associated with gastric cancer differentiation and its deficiency correlates with poor survival of patients after tumor surgical resection (2).
But how Dp71 works as a putative tumor suppress gene in gastric cancer? Based on the previous research of Dp71, the nuclear cytoskeleton protein laminB1 was proved to be a potential down stream target molecule. In one aspect, the concomitant reduction of lamin B1 and Dp71 in gastric cancer and cancer cell lines described in our paper and previous publications confirmed the potential tumor suppressive role of lamin B1. Also other key researches in gastric cancer tissue and cell lines support our hypothesis (3,4).
As an important cytoplasmic and nuclear scaffold protein, Dp71 associates with several dystrophin associated proteins to constitute a nuclear and cytoplasmic DAPC.
As a dominant cytoplasmic isoform, Dp71f in HEK293 cells, bipolar GABAergic and multipolar Glutamatergic neurons were also able to form a nuclear DAPC complex (5,6). Both Dp71f and Dp71d were proved to interact with nuclear envelope proteins and formed nuclear DAPCs, which is involved in the maintenance of nuclear architecture and homoeostasis function of nucleus. In addition to the traditional Dp71 associated proteins such as dystrobrevin, syntrophin, Dp71 was also proved to be able to interact with additional nuclear proteins, such as emerin and lamin B1 to constitute a nuclear envelope protein complex (Figure 1).
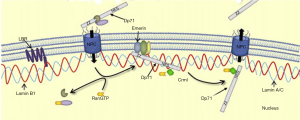
In our publication in Oncotarget (2016), we predicted that the concurrent deficiency of both Dp71 and lamin B1 could alter mitosis progression, which might ultimately lead to chromosomal instability, and finally result in the tumorigenesis. Other NE (nuclear envelope) protein such as emerin might also play an important role in the gastric tumorigenesis, however.
Emerin is a member of LEM-domain family of proteins that localize predominantly at the NE inner membrane. Emerin binds structural components of both the NE (e.g., SUN1, SUN2, nesprins) and the nucleoskeleton (lamins, actin). Emerin plays a role in signaling, mechano-transduction, nuclear architecture, and chromatin tethering and gene regulation (7).
In human ovarian cancer, a significant percentage of emerin loss or abnormal distribution was detected. In human ovarian surface epithelial cells, suppression of emerin leads to nuclear morphological deformation and aneuploid cells (8). In C elegans, after the knocking down of emerin, severe consequences such as mitotic chromosome segregation and postmitotic nuclear assembly were displayed. The interaction between emerin and an essential chromatin protein BAF may play an important role in the aneuploid formation. Located on the so-called “core” regions of anaphase chromosomes at the earliest stages of nuclear assembly, BAF is essential for chromosome segregation, cell cycle progression and post-mitotic nuclear assembly (9).
In addition to its biological function of chromosome segregation, emerin is able to block or attenuate the nuclear accumulation of at least three signaling proteins: ERK1/2, Lmo7 and β-catenin. The activation of the three signaling proteins was reported to play an important role in the tumorigenesis. In emerin-null fibroblasts, β-catenin was increased and accumulated in the nucleus, which finally resulted in increased proliferation of these human fibroblasts. Lmo7 is a transcription factor that activates many genes including the emerin gene, and its accumulation appears to be inhibited by emerin. The nuclear localization and activity of ERK1/2 (a MAPK) increases in emerin-null mouse hearts. The nuclear localization and activity of ERK1/2 (a MAPK) increased in emerin-null mouse hearts. Emerin also functions as a negative regulator of gene expression by trapping transcriptional activators at the nuclear membrane (7). Endogenous emerin inhibition was proved to activate Notch signaling and induce the activation of signaling molecules such as ERK and AKT, which finally promoted cancer cell survival (10).
However, there is no emerin expression description in gastric cancer. Based on the previous research of emerin and lamin B1, it is proposed that the loss of these two nuclear envelopes could act in combination to initiate the chromosome instability and nuclear deformation. Also, the activation of other transcriptional factors and signaling pathway may act in combination to initiate the tumor formation and progression.
Since there are strong correlations between nuclear morphological deformation and malignancy, the functional significance of nuclear morphology in cancer development and progression was speculated about. Both emerin and lamin B1 are major components of NE-associated nucleoskeleton structure nuclear “lamina” (11). Being a direct binding partner of lamin B1 and emerin, knocking down Dp71 in A549 cells and GES-1 cells, a strongly deformed nuclear envelope was observed (unpublished data). Whether the reduction of Dp71 results in tetraploidy/aneuploidy cells needs further cytogenetic analysis, however.
The NE (nuclear envelope) membrane proteome is large and diverse, that includes both ubiquitous and potentially cell type-specific (“unique”) proteins. Different subsets of NETs (nuclear envelope transmembrane) contribute to spatial control of the genome, cell cycle regulation, or cytoskeleton organization, through unknown mechanisms. Maybe the Dp71 “proteomes” will unveiled other nuclear binding partners of Dp71. Techniques such as immunoprecipitation-Mass spectrometry analyses can be used to identify other nuclear interaction proteins. Function together with emerin and lamin B1, these unknown nuclear proteins may act in combination to initiate the tumor formation and progression.
Acknowledgments
Funding: The National Natural Science Fund of China (Grant No. 30800550) and Hunan Natural Science Fund of China (Grant No.10JJ4016).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Xiangya Medicine. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2017.03.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tadayoni R, Rendon A, Soria-Jasso LE, et al. Dystrophin Dp71: the smallest but multifunctional product of the Duchenne muscular dystrophy gene. Mol Neurobiol 2012;45:43-60. [Crossref] [PubMed]
- Tan S, Tan J, Tan S, et al. Decreased Dp71 expression is associated with gastric adenocarcinoma prognosis. Oncotarget 2016;7:53702-11. [PubMed]
- Shimi T, Butin-Israeli V, Adam SA, et al. The role of nuclear lamin B1 in cell proliferation and senescence. Genes Dev 2011;25:2579-93. [Crossref] [PubMed]
- Moss SF, Krivosheyev V, de Souza A, et al. Decreased and aberrant nuclear lamin expression in gastrointestinal tract neoplasms. Gut 1999;45:723-9. [Crossref] [PubMed]
- Nishida A, Yasuno S, Takeuchi A, et al. HEK293 cells express dystrophin Dp71 with nucleus-specific localization of Dp71ab. Histochem Cell Biol 2016;146:301-9. [Crossref] [PubMed]
- Rodríguez-Muñoz R, Cárdenas-Aguayo Mdel C, Alemán V, et al. Novel Nuclear Protein Complexes of Dystrophin 71 Isoforms in Rat Cultured Hippocampal GABAergic and Glutamatergic Neurons. PLoS One 2015;10:e0137328. [Crossref] [PubMed]
- Berk JM, Tifft KE, Wilson KL. The nuclear envelope LEM-domain protein emerin. Nucleus 2013;4:298-314. [Crossref] [PubMed]
- Capo-chichi CD, Cai KQ, Testa JR, et al. Loss of GATA6 leads to nuclear deformation and aneuploidy in ovarian cancer. Mol Cell Biol 2009;29:4766-77. [Crossref] [PubMed]
- Haraguchi T, Koujin T, Osakada H, et al. Nuclear localization of barrier-to-autointegration factor is correlated with progression of S phase in human cells. J Cell Sci 2007;120:1967-77. [Crossref] [PubMed]
- Lee B, Lee TH, Shim J. Emerin suppresses Notch signaling by restricting the Notch intracellular domain to the nuclear membrane. Biochim Biophys Acta 2017;1864:303-13.
- Simon DN, Wilson KL. The nucleoskeleton as a genome-associated dynamic 'network of networks'. Nat Rev Mol Cell Biol 2011;12:695-708. [Crossref] [PubMed]
Cite this article as: Tan S, Xiao X. Dp71 as a tumor suppressor: combined efforts of nuclear lamin B1 and emerin? J Xiangya Med 2017;2:25.


