
Low-dose interleukin-2 as a regulatory immunotherapy for systemic lupus erythematosus
Accumulating evidence that interleukin-2 (IL-2) is requisite for the maintenance and expansion of regulatory T cells (Treg) has yielded low-dose IL-2 therapy for several autoimmune diseases such as type 1 diabetes and systemic lupus erythematosus (SLE) (1,2). He and colleagues recently reported in Nat Med the efficacy of low-dose IL-2 treatment in 38 active SLE patients (3). They described that IL-2 treatment significantly ameliorated the clinical severity associated with expansion of Treg and decrease of follicular helper T (TFH) cells and IL-17-producing helper T (TH17) cells.
IL-2 was first identified as a critical growth factor of T lymphocytes. High-dose IL-2 therapy in melanoma and renal cell carcinoma has been used for more than 20 years in anticipation of expansion of anti-cancer lymphocytes and natural killer cells that are activated and expressed CD25, a high affinity receptor of IL-2. Because IL-2 also plays a critical role for the expansion and function of Tregs, IL-2 supplementation therapy was tried first in mice with type 1 diabetes and revealed that low-dose IL-2 could prevent diabetes progression with significant expansion of Tregs, but high-dose IL-2 did not (4). Shortly thereafter, low-dose IL-2 therapy emerged for the treatment of several human autoimmune and autoimmune-related diseases, and demonstrated selective expansion of Treg cell population associated with clinical response in patients with chronic graft versus host disease (cGVHD) and with chronic hepatitis C-related vasculitis (5,6). In cGVHD, 0.3 to 3 million IU (MIU) of IL-2 were injected subcutaneously daily and the cumulative dosage of IL-2 was 32 to 320 MIU. Treg increase was 8 times higher than controls and NK cells were 2 times higher (5,7). For HCV-associated vasculitis, 1.5 to 3 MIU for 5 days daily for four cycles and up to 50 MIU was used and the therapy led to substantial clinical improvement in both cryoglobulinemia and vasculitis.
In this study by Li and colleagues, 1 MIU IL-2 was administered alternate-day for 7 times at three cycles to active SLE patients. Cumulative dosage was 21 MIU. During and after the course of IL-2 administration, they estimated clinical responses with SLE response index (SRI)-4 response rate (SRI with a 4-point drop in the SLE activity index (SLEDAI)) and the Safety of Estrogens in Lupus Erythematosus National Assessment version of the SLE disease activity index (SELENA-SLEDAI). Patients were from 18 to 65 at their age without severe chronic liver, kidney, lung, heart dysfunction, infection or cancer. Patients were treated with immunosuppressive agents including corticosteroids for at least 4 weeks and not with high-dose steroid pulse therapy in the last 2 months. Seventeen patients were treated with steroid and hydroxychloroquine (HCQ), 13 with steroid, HCQ and mycophenolate mofetil (MMF) and 7 with steroid, HCQ and cyclophosphamide. Although 2 among 40 patients withdrew before the completion of therapy for non-medical reasons, no serious adverse events or bacterial infections were observed during the 12-week period of treatment. At the end of treatment, 89.5% of patients achieved SRI-4 response rate and SELENA-SLEDAI score was significantly decreased. At week 12, 91.9% of corticosteroid-treated patients could reduce prednisone at a dose by more than 25%.
Low-dose IL-2 therapy in SLE was reported first by Humrich and colleagues in 2015 (8). In that case, subcutaneous injection of 1.5 to 3 MIU IL-2 on 5 consecutive days resulted in disappearance of skin eruption, myositis and reduction of serum anti-ds DNA antibody. SLEDAI score decreased from 14 to 4 and glucocorticoid could be reduced from 30 to 10 mg/day. Percentage of CD4+CD25+Foxp3+CD127lo Treg among CD4+ T cells was transiently upregulated at more than 40%. In the current study by Li and colleagues, they also analyzed peripheral Treg, TH1, TH2, TH17 and TFH cells in blood of 23 patients by flow cytometry. Relative Treg number was significantly increased after 12 weeks of IL-2 treatment and TH17 and TFH were decreased, whereas TH1 and TH2 percentages were not changed. IL-2 is reported to suppress the differentiation of CD4+ T cells into TH17 cells and TFH cells by a STAT5-dependent mechanism (9,10). Moreover, IL-2 can expand CD4+ PD-1+ CXCR5+Foxp3+ follicular regulatory cells (TFR) and regulate the balance between TFH and TFR leading to the suppression of germinal center formation (11). Therefore, IL-2 could affect the number of TH17 and TFH cells. Although IL-2 is known to promote the development of TH1 and TH2 cells, low-dose administration of IL-2 might not attain the effect on those cells because of lower expression of IL-2 receptors in these cells than Treg (12). Notably, they also assessed the number of T cell receptor (TCR)αβ+CD4–CD8– double negative (DN) T cells and found that the number was significantly decreased after IL-2 treatment. DN T cells, derived from unknown origin, are known to be expanded in blood from SLE and to favorably produce IL-17 (13). Reduction of DN T cell population by IL-2 also could be involved in the alleviation of disease severity (Figure 1).
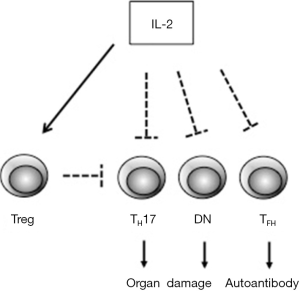
Low-dose IL-2 treatment for SLE could be a promising, selective therapeutic strategy. However, a number of issues remain to be elucidated. First, could low-dose IL-2 be an alternative to immunosuppressive agents? The conclusion still has a long way to go. In all clinical trials, IL-2 administration was performed as an additional therapy to glucocorticoid and/or other immunosuppressive drugs. Conventional therapy itself has some effect on SLE disease activity and it is unknown whether IL-2 has a specific effect on disease regression or not. Thus far, there are no randomized controlled trials of low-dose IL-2 treatment and further examinations are necessary. It should be noted that the study of He et al. (3) compared the results to a control group of patients who were enlisted after the study had added which is unprecedented for clinical trials. Second, how much dosage of IL-2 is required for acquiring substantial effect? IL-2 has a very short half-life in human serum (5–7 minutes) and frequent injection is needed for the induction of effectiveness. The duration of IL-2 treatment could also be a problem. Treg does not seem to increase linearly in response to IL-2 injection after 12 weeks. More studies are needed to define optimal treatment schemes for low-dose IL-2.
Thirdly, the precise mechanisms by which IL-2 alleviates SLE disease severity still remain unclear. Is expanding Treg sufficient for the suppression of autoimmune responses? Lupus T cells have enhanced early CD3/TCR signaling with heightened calcium responses (14). This alteration is in part due to the rewired TCR/CD3 complex. CD3ζ chain is replaced with Fcε receptor 1γ (FcεR1γ, FcRγ) and FcRγ recruits tyrosine kinase Syk instead of Zap70. Nevertheless, IL-2 production of T cells is impaired due to several mechanisms including dephosphorylation of cyclic AMP responsive element binding protein (CREB) through the excessive phosphatase PP2A activity, aberrant expression of cyclic AMP response element modulator (CREM) and decreased expression of serine/arginine-rich splicing factor 1 (SRSF1) (15). Decreased production of IL-2 in SLE patients likely contributes to various immune defects such as reduced Treg, decreased activation-induced cell death and decreased cytotoxic T cell responses. Aside from the effect of IL-2 on Treg expansion, it is also important to verify the restoration of aberrant and/or impaired T cell functions by IL-2 treatment.
Toward future practical usage of low-dose IL-2 for SLE treatment, randomized controlled study and studies involving large number of patients are necessary.
In Europe, phase II trials are now ongoing with other autoimmune and autoinflammatory diseases. IL-2 might be an old new drug for modulating T cell population to shift them suppressive state. In depth evaluation is required to determine whether the modulation of T cell population directly delivers clinical improvement, and to clarify whether long-term administration is safe and effective.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned and reviewed by the Section Editor Yisha Li, MD (Department of Rheumatology, Xiangya Hospital, Central South University, Changsha, China).
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2016.12.15). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol 2015;15:283-94. [Crossref] [PubMed]
- von Spee-Mayer C, Siegert E, Abdirama D, et al. Low-dose interleukin-2 selectively corrects regulatory T cell defects in patients with systemic lupus erythematosus. Ann Rheum Dis 2016;75:1407-15. [Crossref] [PubMed]
- He J, Zhang X, Wei Y, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med 2016;22:991-3. [Crossref] [PubMed]
- Yu A, Zhu L, Altman NH, et al. A low interleukin-2 receptor signaling threshold supports the development and homeostasis of T regulatory cells. Immunity 2009;30:204-17. [Crossref] [PubMed]
- Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 2011;365:2055-66. [Crossref] [PubMed]
- Saadoun D, Rosenzwajg M, Joly F, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med 2011;365:2067-77. [Crossref] [PubMed]
- Matsuoka K, Koreth J, Kim HT, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med 2013;5:179ra43. [Crossref] [PubMed]
- Humrich JY, von Spee-Mayer C, Siegert E, et al. Rapid induction of clinical remission by low-dose interleukin-2 in a patient with refractory SLE. Ann Rheum Dis 2015;74:791-2. [Crossref] [PubMed]
- Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 2007;26:371-81. [Crossref] [PubMed]
- Ballesteros-Tato A, León B, Graf BA, et al. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity 2012;36:847-56. [Crossref] [PubMed]
- Chung Y, Tanaka S, Chu F, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med 2011;17:983-8. [Crossref] [PubMed]
- Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 2012;12:180-90. [PubMed]
- Suárez-Fueyo A, Bradley SJ, Tsokos GC. T cells in Systemic Lupus Erythematosus. Curr Opin Immunol 2016;43:32-38. [Crossref] [PubMed]
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med 2011;365:2110-21. [Crossref] [PubMed]
- Moulton VR, Tsokos GC. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J Clin Invest 2015;125:2220-7. [Crossref] [PubMed]
Cite this article as: Mizui M, Tsokos GC. Low-dose interleukin-2 as a regulatory immunotherapy for systemic lupus erythematosus. J Xiangya Med 2016;1:15.


