Successful echo-guided bedside placement of Impella 2.5 L in a patient with profound cardiogenic shock already supported by intra-aortic balloon pump
Introduction
Cardiogenic shock (CS) is the most common cause of death in patients hospitalized with acute myocardial infarction. It accounts for a high 30-day mortality rate up to 60–80% (1,2). Impella 2.5 (IMP) and intra-aortic balloon pump (IABP) are common mechanical circulatory devices that are used in CS. IABP decreases cardiac afterload, improve coronary perfusion and augments mean arterial pressure (MAP) (3). IMP on the other hand, continuously pumps the blood directly from the left ventricle (LV) to aorta, which decreases LV workload and O2 consumption, subsequently shifting pressure-volume loop to left (4). This further improves hemodynamics including aortic pressure, coronary perfusion, and decreases left atrial as well as pulmonary wedge pressures (4).
Their individual use has been associated with improved hemodynamics in CS without a 30-day mortality benefit (5,6). However, given the lack of a mortality benefit with single strategy of IABP or IMP, combined use of both devices has been suggested and reported in a few cases in literature. To date, no randomized clinical trials have evaluated the benefit of combining both devices for CS treatment (3,5,7-9). In this report, we present a case utilizing both devices, IABP and IMP, to treat CS.
Case presentation
A 42-year-old male with past medical history of diabetes mellitus, hypertension and hyperlipidemia was admitted under the diagnosis of non-ST elevation myocardial infarction (NSTEMI) and severely reduced left ventricular function with ejection fraction of 20%. Coronary angiogram showed severe multi-vessel disease and the patient underwent emergent coronary artery bypass surgery with four vein grafts. His postoperative course was complicated by successful resuscitation of cardiopulmonary arrest and CS requiring vasopressors as well as IABP support.
Despite these measures, the patient continued to deteriorate and the decision was made to add more mechanical circulatory support with IMP. The procedure was performed in the intensive care unit under transesophageal echocardiography (TEE) guidance, after obtaining informed consent, giving to the patient’s instability for transportation to the cardiac catheterization lab.
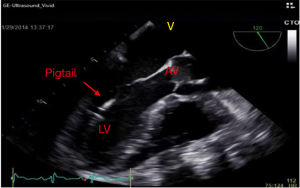
Under standard sterile technique, a right femoral artery access was obtained. IABP on the left side was turned off and a 5 French (Fr) pigtail catheter was advanced over the J wire into the ascending aorta and across the aortic valve (AV) confirmed via TEE (Figure 1). A 0.018” 260 cm wire was placed into LV cavity (Figure 2). Pigtail catheter was removed and ABIOMED IMP was prepped and advanced over the 0.018” wire (Figure 3A).
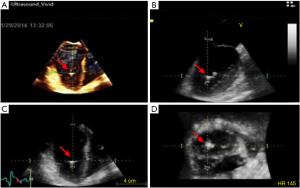
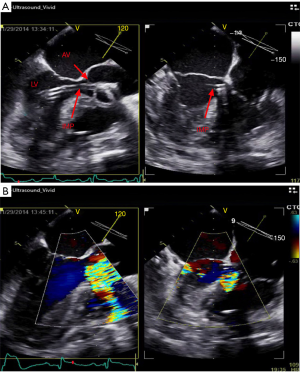
Under TEE guidance, IMP positioning was confirmed and it demonstrated good flow at 2.4 L/minute (Figure 3B). IABP was resumed as well. The 13 Fr peel-away sheath was removed and the repositioning unit inserted into the right femoral artery. Good hemostasis was achieved at the insertion site. Sheath was sutured and secured in place with a sterile dressing. At the end of the procedure, distal pulses were recognized by Doppler ultrasound and had excellent signal.
The patient tolerated the procedure well with rapid improvement of the hemodynamics immediately following IMP placement including mean blood pressure MAP. He continued to improve clinically over the following days. Vasopressor/Inotropic support was gradually weaned off; IABP was discontinued after 48 hours and IMP was pulled out after 72 hours. The patient was finally discharged home after a prolonged hospital stay.
Discussion
IABP and IMP have different mechanisms of action; it has been suggested that they can potentially work together synergistically in patients with refractory CS who need more hemodynamic support than what either device would provide. This synergy would not only optimize hemodynamics, but also may overcome the limitations of each device (10,11). These effects were initially shown in Sauren and colleagues who studied the combined strategy in an animal model (10). This was followed by few case reports where both devices used simultaneously showed superior hemodynamic support compared to single device use (11-13).
The duration of the low output phase is indeed the main determinant of multiple organ failure, so more aggressive unloading of the failing heart in combination with revascularization might improve the outcome (13). Unlike other reported cases in literature, this case showed that under emergent situation, these mechanical support devices can be placed bedside under TEE guidance omitting the need for transportation to Catheterization lab and for fluoroscopy which would safe time in comparison to surgically placed devices and extra-corporeal membrane oxygenation which are more complex and time consuming. In these combination approach cases, there were no significant vascular complications reported, indicating the safety of this strategy. To our knowledge, this is first reported case in literature where IMP was inserted bedside under TEE guidance, which made it very challenging.
In conclusion, IMP placement at bedside under TEE guidance with preexisting contralateral IABP can be safe and effective for treatment of severe CS refractory to either device to provide further hemodynamic support. Further studies are needed to evaluate the efficacy, safety and cost of this approach for treatment of refractory CS.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2016.12.07). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). All authors were involved in the treatment of the patient in this case at the University of Missouri Hospital. Due to the anonymity of the patient presented, no consent was required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khalid L, Dhakam SH. A review of cardiogenic shock in acute myocardial infarction. Curr Cardiol Rev 2008;4:34-40. [Crossref] [PubMed]
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1999 guidelines for the management of patients with acute myocardial infarction). J Am Coll Cardiol 2004;44:671-719. [Crossref] [PubMed]
- Pavlidis AN, Redwood SR, Clapp BR. Combined hemodynamic support with the Impella 2.5 device and intra-aortic balloon pump for management of refractory cardiogenic shock. J Invasive Cardiol 2014;26:E50-1. [PubMed]
- Burkhoff D, Sayer G, Doshi D, et al. Hemodynamics of Mechanical Circulatory Support. J Am Coll Cardiol 2015;66:2663-74. [Crossref] [PubMed]
- Cheng JM, den Uil CA, Hoeks SE, et al. Percutaneous left ventricular assist devices vs. intra-aortic balloon pump counterpulsation for treatment of cardiogenic shock: a meta-analysis of controlled trials. Eur Heart J 2009;30:2102-8. [Crossref] [PubMed]
- Thiele H, Zeymer U, Neumann FJ, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med 2012;367:1287-96. [Crossref] [PubMed]
- Wiktor DM, Sawlani N, Kanthi Y, et al. Successful combined use of Impella Recover 2.5 device and intra-aortic balloon pump support in cardiogenic shock from acute myocardial infarction. ASAIO J 2010;56:519-21. [Crossref] [PubMed]
- Gupta A, Allaqaband S, Bajwa T. Combined use of Impella device and intra-aortic balloon pump to improve survival in a patient in profound cardiogenic shock post cardiac arrest. Catheter Cardiovasc Interv 2009;74:975-6. [Crossref] [PubMed]
- Cubeddu RJ, Lago R, Horvath SA, et al. Use of the Impella 2.5 system alone, after and in combination with an intra-aortic balloon pump in patients with cardiogenic shock: case description and review of the literature. EuroIntervention 2012;7:1453-60. [Crossref] [PubMed]
- Sauren LD, Accord RE, Hamzeh K, et al. Combined Impella and intra-aortic balloon pump support to improve both ventricular unloading and coronary blood flow for myocardial recovery: an experimental study. Artif Organs 2007;31:839-42. [Crossref] [PubMed]
- Pieri M, Contri R, Winterton D, et al. The contemporary role of Impella in a comprehensive mechanical circulatory support program: a single institutional experience. BMC Cardiovasc Disord 2015;15:126. [Crossref] [PubMed]
- Meyns B, Dens J, Sergeant P, et al. Initial experiences with the Impella device in patients with cardiogenic shock - Impella support for cardiogenic shock. Thorac Cardiovasc Surg 2003;51:312-7. [Crossref] [PubMed]
- Bautista-Hernández V, Gutiérrez F, Pinar E, et al. Initial experience with the Impella left ventricular assist device for postcardiotomy cardiogenic shock and unprotected left coronary artery angioplasty in patients with a low left ventricular ejection fraction]. Rev Esp Cardiol 2007;60:984-7. [PubMed]
Cite this article as: Enezate TH, Aggarwal K, Chan A, Balla S, Omran J. Successful echo-guided bedside placement of Impella 2.5 L in a patient with profound cardiogenic shock already supported by intra-aortic balloon pump. J Xiangya Med 2016;1:7.