
Effects of salidroside on myocardial cell apoptosis in acute myocardial ischemia rats and its mechanism
Introduction
Ischemic heart disease is a common cardiovascular disease. With the acceleration of population aging, the prevalence of ischemic heart disease has increased annually. Thus, enhanced prevention and treatment of myocardial ischemia has become a hot research topic in the field of cardiovascular diseases (1). Clinical research and animal experiments (2) have demonstrated that, in human beings, rabbits, and rats, long-term myocardial ischemia can lead to obvious apoptosis of myocardial cells, which is followed by necrosis. Apoptosis, or the process of programmed cell death, is regulated by multiple genes, which mainly include pro-apoptotic gene Bax and antiapoptosis gene Bcl-2; also, the imbalance between these two genes can lead to the transposition of downstream Cytochrome c (Cyt-c) and activation of caspase pathway (3). Many active substances from natural products have anti-myocardial ischemia effect and thus may play certain roles in the prevention and treatment of ischemic cardiomyopathy such as myocardial infarction. Salidroside is the main active ingredient of Tibetan Rhodiola. Although it has been described that salidroside can reduce the area of myocardial infarction, improve myocardial ischemia-reperfusion injuries, and have certain anticoagulation effectiveness (4), most studies were focused on its mechanisms based on its anti-hypoxia and anti-oxygen free radicals capabilities, whereas few studies have explored whether salidroside could fight against myocardial cell apoptosis. Thus, in our current study, we explored the effect of salidroside in suppressing myocardial cell apoptosis and investigated its underlying mechanism, with an attempt to provide laboratory evidences for the clinical application of salidroside.
Materials and methods
Materials and reagents
Salidroside was purchased from the National Institute for the Control of Pharmaceutical and Biological Products. Healthy Sprague-Dawley (SD) rats were purchased from Shanghai SLAC Laboratory Animal Co. Ltd. Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) kit was purchased from Roche, USA. Mitochondrial isolation kit, phosphatase inhibitor Cocktail, and cell lysate RIPA were purchased from Thermo, USA. The primary antibodies Bax, Bcl-2, Cyt-c, cleaved caspase-3, cleaved caspase-9, β-actin, and horseradish peroxidase-labeled goat anti-mouse secondary antibody were purchased from CST, USA.
Establishment of rat models of acute myocardial ischemia (AMI)
The rats were anesthetized using 100 mg/kg ketamine. The thoracic cavity and pericardium were opened layer by layer via the left fifth rib to expose the heart. The left anterior descending coronary artery was ligated using 7–0 silk suture 2 mm below the left atrial appendage. The left ventricular wall turned white after ligation. Then, the lungs were fully expanded to expel the thoracic air before the incisions were closed layer-by-layer. The sham group only received simple open-chest surgery, during which only silk suture was threaded and no ligation was performed.
Animal grouping
Eighty SD rats weighing 200–250 g were equally randomized into four groups: sham group, AMI group, salidroside high-dose (40 mg/kg) group (AMI + HS), and salidroside low-dose (10 mg/kg) group (AMI + LS). Human dose was converted into animal dose based on body surface area, and the drug was intragastrically administered. In the AMI + HS group and AMI + LS group, salidroside (40 or 10 mg/kg) was administered 24 hours after modeling. Equal doses of saline solution were administered in the AMI group. The drug or saline was intragastrically administered daily for two consecutive weeks. The survival rates of the rats were calculated.
Detection of myocardial cell apoptosis
The animals were sacrificed two weeks after surgery in each group. The heart was harvested and fixed in 4% formaldehyde overnight and then embedded in paraffin. Section-cutting was performed along the left ventricular axis at the edge of the myocardial ischemia lesion. The sections were dewaxed in xylene and then put in 100%, 90%, and 70% ethanol for 3 min each before they were rehydrated in distilled water. The sections were washed with phosphate buffer solution (PBS) twice, added with TUNEL solution, inoculated at dark at 37 °C for 30 minutes, incubated in 4’,6-diamidino-2-phenylindole (DAPI) for 5 min, and then immediately put under fluorescence microscope for observation and photo-taking.
Immunoblot (Western blot) for determining protein expressions of relevant genes
The heart tissues were dissected on ice and lysed in RIPA extraction buffer containing 1% cocktail and then incubated on ice for 30 min. After centrifugal separation at 12,000 rpm at 4 °C for 15 min, the supernatant (total cell protein) was collected. After the loading volume was adjusted to the same volume, SDS-PAGE gel electrophoresis was performed. The products were placed in electrophoresis trough, in which the Western transfer was maintained 90 min under a constant current of 200 mA. Then the membrane was blocked in hydrochloride buffer solution containing 5% skimmed milk powder for 1h. The corresponding primary antibody was inoculated at 4 °C overnight; then, the secondary antibody was inoculated at room temperature for 1h. The color reaction was detected using electrogenerated chemiluminescence (ECL) method and exposed on film, and analyzed using ImageJ 1.36b software (National Institutes of Health, USA).
Mitochondria isolation
The rats were sacrificed to harvest heart. According to the kit instructions provided by Thermo Company, the cytoplasmic proteins and mitochondria were isolated using the cytoplasmic mitochondria isolation kit. The transposition of Cyt-c, was detected using Western blotting. Mitochondrial complex IV was used as the loading control.
Statistical analysis
All the experimental results were presented as mean ± standard errors (SE). Paired t-test or One-way ANOVA was performed in the SPSS 11.0 software, with P<0.05 as statistically significant.
Results
Effect of salidroside on survival rate of AMI rats
The AMI model was established in 60 SD rats. In the AMI group, 7 of 20 rats died of cardiac failure within two weeks after surgery, and the survival rate was 65%. The survival rates were 75% and 90% in the AMI + LS group and AMI + HS group, which were significantly different from that in AMI group. No rat died in the sham group, and the survival rate was 100%.
Effect of salidroside on myocardial cell apoptosis of AMI rats
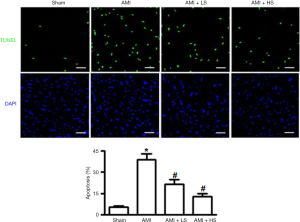
As shown by TUNEL staining, extremely few positively stained cardiac tissues were seen in the sham group, whereas a large number of positively stained cells in AMI group; after salidroside intervention, the number of positively stained cells gradually decreased along with the increased of salidroside dose and showed significant difference with the AMI group (Figure 1).
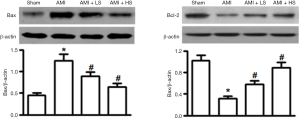
Effect of salidroside on the protein expressions of Bax and Bcl-2
As shown by Western blotting, AMI could significantly increase the protein expression of Bax in cardiac tissue and meanwhile significantly decrease the protein expression of Bcl-2. Salidroside intervention significantly suppressed the increased Bax expression and decreased Bcl-2 expression due to AMI in a dose-dependent manner (Figure 2).
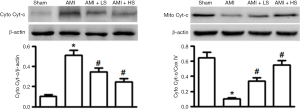
Effect of salidroside on Cyt-c transposition
As shown in Figure 3, compared with the sham group, AMI significantly increased Cyt-c expression in cytoplasma (Cyto) and decreased its expression in mitochondria, suggesting that AMI could induce the Cyt-c’ s transposition from mitochondria to Cyto. Salidroside interventions dose-independently decreased Cyt-c expression in Cyto and meanwhile increased Cyt-c expression in Cyto, thus suppressing the transposition.
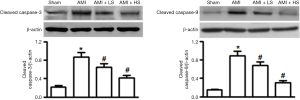
Effect of salidroside on the activation of caspase pathway
As shown in Figure 4, compared with sham group, the protein expressions of cleaved caspase-3 and cleaved caspase-9 significantly increased in AMI group. Salidroside intervention dose-independently decreased the protein expressions of cleaved caspase-3 and cleaved caspase-9, which showed significant difference from those in AMI group.
Discussion
Cell apoptosis is an autonomic ordered programmed cell death in order to maintain homeostasis, which is controlled by serial genes (3). In recent years, myocardial cell apoptosis has become a hot research topic. The role of apoptosis is particularly important during the development of an ischemic heart disease (e.g., myocardial infarction). Research (5) has revealed that apoptosis not only occurs at the centers of infarct lesions but also in the survived myocardium adjacent to or distant from the infarct lesions. The morphologies of apoptotic cells can be observed in different stages, showing unique biological features. One of the main features is that the apoptosis can activate DNA endonuclease, which catalyzes the incision of genomic DNA among nucleosomes and thus expose the 3’-OH of DNA. The 3’-OH can be added with fluorescein-labeled dUTP under the catalyzation of terminal deoxynucleotide transferase (6). Thus, the degree of apoptosis can be determined by measuring the intensity of specific fluorescence. In our current study, the TUNEL staining showed that the salidroside intervention groups had significantly fewer TUNEL-positive cells in myocardial tissues than in AMI group, which provided preliminary evidence that salidroside had certain effect in fighting against myocardial cell apoptosis.
The Bcl-2 family is composed of the anti-apoptosis protein Bcl-2 and pro-apoptosis protein Bax. They play an important role in maintaining mitochondrial integrity and are key regulators of endogenous mitochondrial pathways of apoptosis (7). Bcl-2 can regulate mitochondrial membrane permeability in mammalian cells. Normally most Bcl-2 is located in mitochondrial outer membrane. The pro-apoptotic factor BAX becomes oligomerized after the cells receive the apoptosis signal; it is transferred from cytoplasm to mitochondrial outer membrane and interacts with the voltage-dependent anion channel on the membrane, thereby allowing the release of apoptogenic factors including Cyt-c into cytoplasmic matrix and thus causing the death of cells (8). Clearly, regulation of the release of Cyt-c from the mitochondria is a key issue in the study on the molecular mechanism of cell apoptosis. In our current study, salidroside not only suppressed the AMI-induced expression of pro-apoptotic factor Bax but also promoted the expression of anti-apoptotic factor Bcl-2, thus increasing the Bcl-2/Bax ratio and reducing the release of Cyt-c from mitochondria to cytoplasm. It has been found that, during the apoptosis, Cyt-c that has been released to cytoplasm can combine with the apoptotic protease activating factor-1(Apaf-1) to form a polymer in the presence of dATP; meanwhile, it promotes caspase-9 to bind to it to form apoptotic body; then, the successive cleaved activation of caspase-9 and caspase-3 finally induces apoptosis (9). In our current study, Western blotting was applied to detect the expressions of cleaved caspase-3 and caspase-9; the results showed that the caspase-3 and caspase-9 proteins in myocardial tissues were activated during AMI and salidroside could significantly suppress the expressions of these two proteins.
Conclusions
In summary, salidroside has a role in fighting against AMI-induced myocardial cell apoptosis. The possible molecular mechanisms may be as follows: salidroside can promote Bcl-2 expression and meanwhile decrease Bax expression, thus suppressing the release of Cyt-c from the mitochondria to the cytoplasm; as a result, it exerts its effect by inhibiting the activities of the downstream caspase-3 and caspase-9.
Acknowledgments
Funding: This work was supported by Guangdong Natural Science Foundation (S2012040008068, S2013010014514), Fund of Guangdong Province-Ministry of Education Producing, Study and Research Incorporation (2012B091100454) and the Fundamental Research Funds for the Central Universities in China (12ykpy26).
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jxym.2016.12.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study followed the national or institutional guidelines for the care and use of animals. The study was approved by ethics committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University (No. 201405006).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maeder MT, Schoch OD, Rickli H. A clinical approach to obstructive sleep apnea as a risk factor for cardiovascular disease. Vasc Health Risk Manag. 2016;12:85-103. [Crossref] [PubMed]
- Shangguan HJ, Xu J, Tang JQ, et al. Effects of angelica on myocardial apoptosis and expression of apoptosis-associated genes after myocardial infarction. Medical Journal of Wuhan University 2005;26:172-5. In Chinese.
- Green DR, Galluzzi L, Kroemer G. Cell biology. Metabolic control of cell death. Science 2014;345:1250256. [Crossref] [PubMed]
- Li J, Fan WH, Luo XP, et al. Effect of Rhodiola Rosae decotion pre administration to the heat function and Flk-1 mRNA expression in myocardial infraction rat. Fudan University Journal of Medical Sciences 2006;33:384-7. In Chinese.
- Li H, Song F, Duan LR, et al. Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/Akt pathway. Sci Rep 2016;6:23693. [Crossref] [PubMed]
- Loo DT. In situ detection of apoptosis by the TUNEL assay: an overview of techniques. Methods Mol Biol 2011;682:3-13. [Crossref] [PubMed]
- Kvansakul M, Hinds MG. The Bcl-2 family: structures, interactions and targets for drug discovery. Apoptosis 2015;20:136-50. [Crossref] [PubMed]
- Raemy E, Martinou JC. Involvement of cardiolipin in tBID-induced activation of BAX during apoptosis. Chem Phys Lipids 2014;179:70-4. [Crossref] [PubMed]
- Tait SW, Green DR. Mitochondrial regulation of cell death. Cold Spring Harb Perspect Biol 2013;5. [PubMed]
Cite this article as: Li J, Li JF, Wei TT, Li JH. Effects of salidroside on myocardial cell apoptosis in acute myocardial ischemia rats and its mechanism. J Xiangya Med 2016;1:5.


